Abstract
Drug-eluting stents (DES) have gained great popularity because of extraordinarily low rates of restenosis. Despite these superior clinical outcomes, several cases regarding the severe multi-vessel coronary spasm, although rare, after the placement of first generation DES have been reported. We report a case of severe, multi-vessel coronary spasm that occurred two occasions after placement of a zotarolimus-eluting stent, one of the second generation DES, in a 42-year-old man with unstable angina. The first incidence was relieved by intracoronary nitroglycerin alone, and second incident, which had combined fixed stenosis was treated with intracoronary nitroglycerin and everolimus-eluting stent.
The use of drug-eluting stents (DES) has significantly changed the practice of interventional cardiology. Several randomized, controlled clinical trials have demonstrated lower rates of major adverse cardiac events as well as in-stent restenosis in patients with DES than those with bare-metal stents (BMS).1) However, unexpected serious, idiosyncratic reactions, although rare, related to DES with polymer and active pharmacologic constituents remains a concern (such as severe spasm and thrombosis).2) While there are several reports regarding the severe multi-vessel coronary spasm after the placement of first generation DES,3-6) no reports have been published regarding the spasm in relation to second generation DES which were designed for a decrease in neointimal response and more rapid re-endothelialization.7)
Herein, we report a case of severe, multi-vessel coronary spasm that occurred on two occasions, two months and two years after the placement of zotarolimus-eluting stents; first one was relieved by intracoronary nitroglycerin alone and the second was treated with intracoronary nitroglycerin and everolimus-eluting stents.
A 42-year-old man was admitted to Chonnam National University Hospital (Gwangju, Korea) for acute chest pain. He was an ex-smoker with a 20 pack-years history, but he didn't have any other significant past-medical history such as dyslipidemia, diabetes or hypertension. He had been on regular anti-anginal medication over the past two years since he underwent pecutaneous coronary intervention (PCI) with a 2.75×18 mm zotarolimus-eluting stent (Endeavor Resolute®, Medtronic vascular, Santa Rosa, CA, USA) in the mid-left anterior descending artery (LAD) due to unstable angina pectoris (Fig. 1).
Two months after the stent implantation, he developed severe chest pain. The 12-lead electrocardiogram (ECG) on admission showed atrial fibrillation and new onset ST-segment elevation in lead aVR with ST-segment depression in multiple other leads, suggesting a left main coronary artery disease (Fig. 2). During the initial evaluation in emergency room, he developed pulseless ventricular tachycardia, which was successfully resuscitated with electric countershock. A coronary angiogram (CAG) after the resuscitation showed severe multi-vessel spasm with near total occlusion of the proximal LAD, proximal left circumflex artery and mid-right coronary artery (RCA) with a patent previously implanted stent in the mid-LAD (Fig. 3A and B). The spasm was completely relieved by intracoronary nitroglycerin injection (Fig. 3C and D). A calcium channel antagonist and oral nitrate were added to anti-anginal medication after recovery.
Two years after index PCI, he again compained of severe squeezing chest pain despite the continued use of anti-anginal medications including nitrate and calcium channel antagonists. He was again admitted to the hospital. An ECG on admission showed ST depression and T wave inversion on the lateral leads; however, the cardiac enzymes were within normal limits. CAG revealed severe vasospasm in the proximal and distal LAD, and mid-RCA (Fig. 4A and B). Intracoronary administration of nitroglycerin relieved the vasospasm, but there was de novo stenosis in the proximal LAD at the proximal edge of the previously implanted zotarolimus-eluting stent (Fig. 4C). The intravascular ultrasound (IVUS) showed a large amount of plaque (minimal lumen area: 3.7 mm2, plaque burden: 55%), for which stenting was performed using a 3.0×24 mm everolimus-eluting stent (Promus Element, Boston scientific, Natick, MA, USA) (Fig. 4D). The final CAG and IVUS showed good distal flow without residual stenosis (Fig. 5). After an uneventful recovery, he was discharged with medication for coronary vasospasm including a dual calcium channel antagonist, nitrate, and statin.
There have been several clinical reports regarding severe simultaneous multi-vessel spasm associated with life-threatening arrhythmias or electromechanical dissociation after implantation of first generation DES such as sirolimus-eluting (Cypher, Cordis, Miami Lakes, FL, USA) and paclitaxel-eluting stents (Taxus, Boston Scientific, Natick, MA, USA).3-6) However, there is paucity of reports regarding severe multivessel coronary spasm after single vessel stenting;3) moreover, no report has been documented on second generation DES. To our best knowledge, this is the first case in which the patient suffered from recurrent catastrophic simultaneous multi-vessel coronary spasms after implantation of a zotarolimus-eluting stent in a single vessel despite the continued medical treatment for coronary vasospasm.
Although the precise mechanisms underlying the multi-vessel spasm after DES implantation in patients with coronary stenosis have not yet fully elucidated, hypersensitivity reactions to stent components or anti-platelet agents manifested as endothelial dysfunction, namely Kounis syndrome have been recognized as key components of spasm.4)8) Several components including: the polymer coating, the drug (i.e., rapamycin and paclitaxel) or the stainless steel components could be responsible for the hypersensitivity reaction. However, the stainless-steel platform is less likely to be the causative factor, given the large number of BMS deployed worldwide and the paucity of similar reports of diffuse spasm. Moreover, BMS failed to demonstrate a hypereosinophilic, immunoglobin E-mediated reaction in human autopsies of over 400 stents.9) The drug reaction to zotarolimus is less likely, because pharmacokinetic studies performed in dogs and rabbits showed that the drug was undetectable in the arterial wall by 60 days after stent implantation.10) As severe multi-vessel spasm occurred 2 months and 2 years after the placement of DES in our case, the most likely etiology of the spasm is the hypersensitivity reaction to the polymer coating of the DES which is known cause a more persistent and prolonged reaction. Indeed, Byrne et al.11) recently had demonstrated that a durable polymer carrier plays a significant role in the DES related hypersensitivity reaction and delayed vessel healing and has pro-inflammatory and thrombogenic potential. Another possible etiology of our case is that the patient had underlying multi-vessel spastic angina with coexisting fixed atherosclerotic disease, and the spasm component had manifested after stent implantation. The incidence of documented diffuse three vessel spasm by intravenous ergonovine was 16.1% in a study performed in Korea12) and multivessel spasm (spasm on more than 2 epicardial arteries) was 49.7% in a study performed in Japan.13) In Northeast Asia, diffuse multi-vessel spasm is not a rare phenomenon in patients with vasospastic angina and is well controlled with medications and giving thema good prognosis similar to patients having previously known vasospastic angina.14) Nevertheless, spontaneous simultaneous multiple coronary artery spasm causing multi-site myocardial infarction, cardiogenic shock, and ventricular fibrillation is extremely rare and there is only one case report in English-language medical literature.15)
Our case had intractable vasospastic angina, defined as an angina that could not be controlled even with the combined administration of two types of coronary vasodilators (i.e., calcium channel antagonist and nitrate), which occurs in 13.7% of vasospastic angina patients.16) Currently, intractable vasospastic angina is treated with a combination of different classes of calcium channel blockers and nitrates or nicorandil or both. In addition to these medications, magnesium,17)18) antioxidants,19) and statins20) were shown to provide additional anti-spasmotic activity. Further, as the atherosclerotic process is an important mechanism of vessel spasm, a detailed evaluation of the amount and distribution of atherosclerotic plaque burden by using IVUS could provide additional treatment option such as coronary artery stenting.21)
Patients with multi-vessel coronary artery spasm often develop lethal arrhythmia such as ventricular tachycardia or fibrillation. These arrhythmias can be managed with the implantation of cardioverter defibrillator, although its use is still controversial.22) To prevent this catastrophic multi-vessel vasospasm, Kounis et al.2) has recommended antibody testing and intradermal skin tests for every stent component where appropriate before the stent insertion, and the monitoring of the level of inflammatory mediators immediately after the insertion.
Our case suffered recurrent sudden chest pain due to severe multi-vessel spasm despite continued anti-anginal therapy, probably related to the implanted DES. On his second attack, showing combined fixed stenosis in proximal LAD, one may have agonized over whether to only use plain balloon angioplasty or implant a BMS instead of a DES in view of known hypersensitive reaction to the components.23) However, the lesion was treated with another second generation DES, everolimus-eluting stent, because this stent was shown to be faster in terms of re-endothelialization process as compared with the other second generation DES such as sirolimus-eluting, paclitaxel-eluting, or zotarolimus-eluting stents24) and to have low risk of restenosis and stent thrombosis.25)
In conclusion, the clinician should be alerted to this potential risk of augmented multi-vessel spasm even with second generation DES.
Figures and Tables
Fig. 1
Coronary angiogram at first admission. A: significant stenosis (arrow) in mid left anterior descending artery (LAD) (right anterior oblique cranial view). B: no significant stenosis in right coronary artery (left anterior oblique caudal view). C: good distal flow after drug-eluting stent implantation (2.75×18 mm Endeavor-R stent) in the mid LAD artery (right anterior oblique cranial view).

Fig. 2
Twelve-lead electrocardiogram showed atrial fibrillation and ST-segment elevation in lead aVR with ST-segment depression in multiple other leads, suggesting left main coronary artery disease.
Fig. 3
Coronary angiogram at on second admission. A: spastic near total occlusion in proximal left anterior descending (LAD), proximal left circumflex artery (arrows) with a patent previously implanted stent in mid LAD (right anterior oblique cranial view). B: mid right coronary artery showing spastic near total occlusion (left anterior oblique caudal view) (arrow). C and D: the spasm was completely relieved by intracoronary nitroglycerin injection.
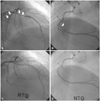
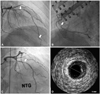
Fig. 4
CAG and IVUS at last admission (i.e., third admission). A: spasmodic stenosis in proximal and distal LAD (arrows). B: mid right coronary artery showing spasmodic stenosis (arrow). C: a repeat CAG after intracoronary administration of nitroglycerin demonstrated fixed stenosis in the proximal LAD at the proximal edge of the previously implanted zotarlimus-eluting stent (arrow). D: IVUS showing a large amount of plaque (minimal lumen area: 3.7 mm2, plaque burden: 55%). CAG: coronary angiography, IVUS: intravascular ultrasound, LAD: left anterior descending artery.
References
1. Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002. 346:1773–1780.
2. Kounis NG, Kounis GN, Soufras GD. Kounis syndrome: a potential cause of simultaneous multivessel coronary spasm and thrombosis after drug-eluting stent implantation. J Invasive Cardiol. 2007. 19:200–201.
3. Tomassini F, Varbella F, Gagnor A, Infantino V, Luceri S, Conte MR. Severe multivessel coronary spasm after sirolimus-eluting stent implantation. J Cardiovasc Med (Hagerstown). 2009. 10:485–488.
4. Brott BC, Anayiotos AS, Chapman GD, Anderson PG, Hillegass WB. Severe, diffuse coronary artery spasm after drug-eluting stent placement. J Invasive Cardiol. 2006. 18:584–592.
5. Togni M, Eberli FR. Vasoconstriction and coronary artery spasm after drug-eluting stent placement. J Invasive Cardiol. 2006. 18:593.
6. Kim JW, Park CG, Seo HS, Oh DJ. Delayed severe multivessel spasm and aborted sudden death after Taxus stent implantation. Heart. 2005. 91:e15.
7. Butt M, Connolly D, Lip GY. Drug-eluting stents: a comprehensive appraisal. Future Cardiol. 2009. 5:141–157.
8. Togni M, Windecker S, Cocchia R, et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol. 2005. 46:231–236.
9. Tan W, Cheng KL, Chen QX. Hypersensitivity to drug-eluting stent and stent thrombosis: Kounis or not Kounis syndrome? Chin Med J (Engl). 2009. 122:2390–2393.
10. Suzuki T, Kopia G, Hayashi S, et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001. 104:1188–1193.
11. Byrne RA, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009. 57:567–584.
12. Koh KK, Moon TH, Song JH, et al. Comparison of clinical and laboratory findings between patients with diffuse three-vessel coronary artery spasm and other types of coronary artery spasm. Cathet Cardiovasc Diagn. 1996. 37:132–139.
13. Sugiishi M, Takatsu F. Cigarette smoking is a major risk factor for coronary spasm. Circulation. 1993. 87:76–79.
14. Park YM, Han SH, Ko KP, et al. Diffuse multi-vessel coronary artery spasm: incidence and clinical prognosis. Int J Cardiol. 2012. [Epub ahead of print].
15. Chuang YT, Ueng KC. Spontaneous and simultaneous multivessel coronary spasm causing multisite myocardial infarction, cardiogenic shock, atrioventricular block, and ventricular fibrillation. Circ J. 2009. 73:1961–1964.
16. Kusama Y, Kodani E, Nakagomi A, et al. Variant angina and coronary artery spasm: the clinical spectrum, pathophysiology, and management. J Nippon Med Sch. 2011. 78:4–12.
17. Miyagi H, Yasue H, Okumura K, Ogawa H, Goto K, Oshima S. Effect of magnesium on anginal attack induced by hyperventilation in patients with variant angina. Circulation. 1989. 79:597–602.
18. Teragawa H, Kato M, Yamagata T, Matsuura H, Kajiyama G. The preventive effect of magnesium on coronary spasm in patients with vasospastic angina. Chest. 2000. 118:1690–1695.
19. Motoyama T, Kawano H, Kugiyama K, et al. Vitamin E administration improves impairment of endothelium-dependent vasodilation in patients with coronary spastic angina. J Am Coll Cardiol. 1998. 32:1672–1679.
20. Yasue H, Mizuno Y, Harada E, et al. Effects of a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, fluvastatin, on coronary spasm after withdrawal of calcium-channel blockers. J Am Coll Cardiol. 2008. 51:1742–1748.
21. Park YM, Kang WC, Shin KC, et al. Repeated sudden cardiac death in coronary spasm: is IVUS helpful to decide treatment strategy? Int J Cardiol. 2012. 154:e57–e59.
22. Kwon TG, Bae JH, Jeong MH, et al. N-terminal pro-B-type natriuretic peptide is associated with adverse short-term clinical outcomes in patients with acute ST-elevation myocardial infarction underwent primary percutaneous coronary intervention. Int J Cardiol. 2009. 133:173–178.
23. Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006. 47:175–181.
24. Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008. 52:333–342.
25. Sarno G, Lagerqvist B, Carlsson J, et al. Initial clinical experience with an everolimus eluting platinum chromium stent (Promus Element) in unselected patients from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Int J Cardiol. 2012. [Epub ahead of print].




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download