Abstract
Background and Objectives
Ventricular fibrillation (VF) can inadvertently occur during electrophysiologic study (EPS) or catheter ablation. We investigated the incidence, cause, and progress of inadvertently developed VF during EPS and catheter ablation.
Subjects and Methods
We reviewed patients who had developed inadvertent VF during EPS or catheter ablation. Patients who developed VF during programmed ventricular stimulation to induce ventricular tachycardia or VF were excluded.
Results
Inadvertent VF developed in 11 patients (46.7±9.3 years old) among 2624 patients (0.42%); during catheter ablation for atrial fibrillation (AF) in nine patients, frequent ventricular premature beats (VPBs) in one, and Wolff-Parkinson-White (WPW) syndrome were observed in one. VF was induced after internal cardioversion in six AF patients due to incorrect R-wave synchronization of a direct current shock. Two AF patients showed spontaneous VF induction during isoproterenol infusion while looking for AF triggering foci. The remaining AF patient developed VF after rapid atrial pacing to induce AF, but the catheter was accidentally moved to the right ventricular (RV) apex. A patient with VPB ablation spontaneously developed VF during isoproterenol infusion. The focus of VPB was in the RV outflow tract and successfully ablated. A patient with WPW syndrome developed VF after rapid RV pacing with a cycle length of 240 ms. Single high energy (biphasic 150-200 J) external defibrillation was successful in all patients, except in two, who spontaneously terminated VF. The procedure was uneventfully completed in all patients. At a mean follow-up period of 17.4±15.5 months, no patient presented with ventricular arrhythmia.
During the past decade, clinical electrophysiologic studies (EPS) have become an accepted part of the diagnostic evaluation to guide the treatment of patients with various cardiac arrhythmias.1)2) Sometimes, electrophysiologists try to intentionally induce ventricular fibrillation (VF) or ventricular tachycardia (VT) by programmed stimulation so as to diagnose previously undocumented or undiagnosed ventricular arrhythmia in certain patients during EPS.3)4) However, VF can also inadvertently arise during EPS or catheter ablation, albeit rarely, in patients without a history of ventricular arrhythmia. Despite the increasingly wide applications of EPS and catheter ablation, there is little information about the incidence and cause of inadvertently developed VF during EPS.
Therefore, this study was systematically carried out to examine 1) the incidence, cause, and progress of inadvertently developed VF during EPS or ablation and 2) the implications of inadvertently developed VF on the recurrence of cardiac events or ventricular arrhythmia during long-term follow-up among patients undergoing EPS without a history of VF.
We retrospectively reviewed 2624 patients who had undergone EPS and/or catheter ablation from January 2008 to October 2012, and sought those who had developed inadvertent VF during EPS or catheter ablation. Patients who had developed VF during programmed ventricular stimulation for the clinical purpose of inducing VF or VT were excluded. Patients with previously documented ventricular tachyarrhythmia or cardiac arrest were also excluded. A database was constructed using clinical histories, demographic data, methodological details of the procedures, and patient progress.
An EPS was performed without any anti-arrhythmic drugs in all patients after informed consent was obtained. Besides amiodarone, anti-arrhythmic medications were discontinued at least 5 half-lives prior to the procedure. Amiodarone was discontinued at least 1 month prior to the ablation procedure. Only ablation procedures in atrial fibrillation (AF) patients were performed under sedation with intravenous propofol and continuous monitoring of blood pressure and oxygen saturation. Other patients were not sedated. Electrocardiograms (ECGs) were continuously monitored from the time patients entered the laboratory until they returned to the hospital room.
The type of arrhythmia determined the catheter insertion and positioning as well as the protocol of programmed stimulation. Briefly, the high right atrium, low right atrium, and coronary sinus were mapped with a decapolar catheter (Bard Electrophysiology Inc., Lowell, MA, USA) and a steerable duo-decapolar catheter (St. Jude Medical Inc., Minnetonka, MN, USA), while a quadripolar catheter was placed in the superior vena cava during catheter ablation for AF. With paroxysmal supraventricular tachycardia, the low right atrium and coronary sinus were mapped with a steerable duo-decapolar catheter while two quadripolar catheters were placed in the His area and right ventricular (RV) apex, respectively.
Two SL1 long sheaths (St. Jude Medical Inc., Minnetonka, MN, USA), one for the ablation catheter and one for the ring-shaped multielectrode catheter (Biosense Webster Inc., Diamond Bar, CA, USA), were inserted into the RV outflow tract to map frequent ventricular premature beats (VPBs) when the origin was suspected as the RV outflow tract, according to ECG characteristics.
Each patient underwent an initial baseline study, which included an evaluation of the atrial, atrio-ventricular (AV) conduction system, and ventricular electrophysiologic variables. The atrio-His interval and His-ventricular interval were measured during sinus rhythm. Programmed stimulation was performed at twice the diastolic threshold current from the high right atrium and RV apex. The standard protocol of programmed electrical stimulation included: 1) atrial and ventricular pacing at cycle lengths ranging from just under that of the sinus rhythm to 300 ms; 2) single, double, or triple atrial extrastimuli delivered during high right atrial pacing at one or two cycle lengths and during sinus rhythm; and 3) single ventricular extrastimuli delivered during RV apex pacing at cycle lengths of 600 and 450 ms and during sinus rhythm.
The AF triggering pulmonary vein (PV) and non-PV foci were evaluated under high dose isoproterenol infusion (10-20 µg/min) after internal cardioversion in any cases of paroxysmal AF at the beginning of the procedure.
Intracardiac electrograms were recorded using an electrophysiology system (Prucka CardioLab™ General Electric Health Care System Inc., Milwaukee, WI, USA or EP Workmate recording system, EP Medical Systems, Mt. Arlington, NJ, USA). Electrical stimulation was performed with a digital stimulator and optically isolated constant current sources (Bloom Associates, Ltd., Narberth, PA, USA).
Ventricular fibrillation accidentally initiated during EPS or catheter ablation was considered to be inadvertent if it was not clinically related to the patient's indication for study.
All patients were hospitalized for monitoring and discharged after stabilization. Patients were seen in an outpatient clinic at two weeks and 3, 6, 9, and 12 months after the procedure, and then annually, thereafter. A 12-lead surface ECG was performed at every visit. AF patients were evaluated by 24- or 48-hour Holter monitoring at 3, 6, 9, and 12 months. Detailed histories were taken regarding any symptoms suggesting arrhythmia or cardiac events.
Among the 2624 patients, 11 developed inadvertent VF (0.42%). Their mean age was 46.7±9.3 years, and 10 patients (91%) were male. Nine patients (82%) developed inadvertent VF during catheter ablation for AF, one patient developed it during ablation for frequent VPBs, and one patient for Wolff-Parkinson-White (WPW) syndrome. Five patients (No. 4, 5, 6, 8, and 10) presented with hypertension. Patient No. 5 had hypertrophic cardiomyopathy without dynamic outflow obstruction. Patient No. 10 had undergone an implantation of a permanent pacemaker nine years prior to catheter ablation due to sick sinus syndrome. No patient had dilated cardiomyopathy or ischemic cardiomyopathy. All AF patients were treated with antiarrhythmic agents (propafenone in 4 patients, flecainide in 2 patients, sotalol in 2 patients, and pilsicainide in 1 patient) before EPS and catheter ablation (Table 1). Nine AF patients were sedated during EPS, while 2 patients were awake. In all 11 patients, VF was induced during the procedure.
Ventricular fibrillation was induced after internal cardioversion in six AF patients (No. 3, 4, 6, 7, 10, and 11) due to the incorrect R-wave synchronization of direct current (DC) shock, which was delivered at the peak of the T wave as R on T. All patients underwent cardioversion during AF rhythm to evaluate AF triggering PV and non-PV foci under high dose isoproterenol (10 µg/min) infusion. A representative example of VF (Patient No. 4) after incorrect R-wave synchronization of DC shock is as shown in Fig. 1. The previous R-R interval before shock was 439 ms. The QT interval of the previous cardiac cycle and the last QRS to the shock interval were 204 and 177 ms, respectively. The pre-shock coupling interval almost corresponded with the terminal portion of the preceding QT interval. Two AF patients (No. 5 and 8) showed spontaneous VF induction during isoproterenol infusion (Fig. 2). These two patients also received highdose isoproterenol infusion (10 µg/min) to evaluate AF triggering atrial foci. The remaining AF (No. 2) patient developed VF after rapid atrial pacing with a cycle length of 210 ms in an attempt to induce AF, but the catheter was accidentally moved to the RV apex after pacing. Surface ECG showed atrial capture at the beginning of the programmed stimulation, which changed to ventricular capture when VF was induced (Fig. 3). The patient for VPB ablation (No. 1) developed VF spontaneously during infusion with low-dose isoproterenol (3 µg/min). Isoproterenol was infused because spontaneous VPBs were rarely observed at the beginning of the procedure. Soon after isoproterenol infusion, frequent polymorphic non-sustained VT was observed, which spontaneously degenerated into VF (Fig. 4). In this patient, the focus of VPBs was at the RV outflow tract and was successfully ablated. The patient with WPW syndrome (No. 9) developed VF after rapid ventricular pacing with a cycle length of 240 ms. The bypass tract was located at the left lateral AV groove. After defibrillation, the bypass tract was successfully ablated, and arrhythmia was not induced by repeated programmed stimulation at the end of the procedure. In summary, 8 patients (72.7%) developed VF after internal cardioversion or rapid RV pacing, whereas 3 patients (27.3%) developed VF spontaneously. Nine patients required highenergy (biphasic 150-200 J) external defibrillation, and a single shock was successful in restoring sinus rhythm within 32.7±23.5 seconds. VF terminated spontaneously in two patients (No. 5 and 11) within 15 and 3 seconds, respectively. The procedure was completed uneventfully for all patients. Serial ECGs were obtained during the 24 hours following the procedure. Serial ECGs demonstrated no specific ST-T wave changes after defibrillation in all patients. All patients were discharged without any sequelae.
Inadvertent VF that develops during EPS or catheter ablation is very rare, with an incidence rate of 0.42%. In this study, the main cause of inadvertently developed VF was incorrect R-wave synchronization of the DC shock (55%) under isoproterenol infusion, followed by spontaneous VF induction during isoproterenol infusion (27%). Progress after VF was favorable in all patients, and no patients presented with adverse cardiac events or ventricular arrhythmia during follow-up. The current study demonstrates the incidence, cause, and progress of inadvertently developed VF during EPS and ablation over 5 years.
Among 1000 patients previously undergoing EPS, incidental VF not related to a patient's indication for study developed in four patients through programmed stimulation.5) In the previous study, all patients developed VF by double ventricular extrastimuli. It is widely known that VF can develop with programmed ventricular extrastimulation in certain patients.4) In the current study, only two patients (18%) presented with inadvertent VF through programmed ventricular stimulation. The two main causes of inadvertent VF in this study (VF due to incorrect R-wave synchronization of the DC shock and spontaneous VF during isoproterenol infusion), have not been previously reported.
Low-energy internal defibrillation can be used effectively and safely to restore AF to sinus rhythm during an EPS.6)7) It is known that delivering an unsynchronized shock, either externally or internally, can result in VF because of the vulnerable ventricular repolarization period. Isoproterenol infusion can shorten the R-R interval, which increases the relative vulnerable period for ventricular repolarization proportionally. However, the potential risk of inducing ventricular proarrhythmia remains, even after a well-synchronized Rwave shock. Two cases of inadvertently induced VF were reported separately in patients with WPW syndrome after internal defibrillation to terminate AF during EPS.8)9) ECG analysis revealed correct R-wave synchronization in both cases, but a shorter preceding RR interval (252 ms) than the previous beat was shown.8) Another patient had an R-wave synchronized shock after a short-long-short ventricular cycle length pattern with maximal pre-excitation and a pre-shock coupling interval of 245 msec. Moreover, the QT interval during maximal pre-excitation was longer, prolonging the vulnerable period of ventricular repolarization more than for non-preexcited beats, presenting more opportunities for R on T induced VF.
Isoproterenol acts as beta adrenergic stimulation and shortens the ventricular effective refractory period. Therefore, beta adrenergic stimulation with isoproterenol prolonged the duration of fibrillation. The effect of isoproterenol was mediated directly via cardiac beta receptors, rather than indirectly via any heart rate or blood pressure changes.10) Studies in isolated perfused rat hearts, a model that eliminates non-cardiac effects, have found that beta adrenergic stimulation increases ventricular vulnerability.11) In our study, 9 (82%) of 11 patients developed inadvertent VF either spontaneously or after incorrect R-wave synchronization of the DC shock during isoproterenol infusion. This suggests that the increased ventricular vulnerability caused by isoproterenol infusion can lead to VF and is a major cause of inadvertent VF during EPS and catheter ablation. Catheter ablation for AF has become a standard treatment method and is widely implicated as a treatment method in current practice. Therefore, cardioversion for terminating arrhythmia and high-dose isoproterenol infusion to induce arrhythmia are ordinarily performed during an ablation procedure. The total incidence of inadvertent VF during catheter ablation for AF is much higher than during EPS or catheter ablation for other arrhythmia {0.94% (9/957) vs. 0.12% (2/1667)}. Therefore, minimizing inadvertent VF during AF ablation procedures, using the greatest possible level of care, is highly necessary.
In all patients, external patches for defibrillation were prepared before the EPS procedure was undertaken. Defibrillation was applied within 60 seconds in all patients except one. All patients recovered completely without sequelae. VF induced during EPS in patients without structural heart disease or a history of ventricular arrhythmia almost always responds to the prompt application of DC transthoracic shocks. VF that is unresponsive to standard treatment, including repeated external defibrillation, may be treated with an emergency thoracotomy with open chest cardiopulmonary resuscitation, and intrathoracic defibrillation12) or intracardiac defibrillation, which uses a previously inserted standard RV quadripolar catheter as a cathode and a posterior skin patch as an anode.13) All current patients responded with the first shock, and none needed repeated shocks or further treatment. Previously, ischemic or infarcted myocardium,14) type I and type III anti-arrhythmic drug therapy,15)16) and increased transthoracic impedance caused by obesity or chronic obstructive pulmonary disease were reported as decreasing transthoracic defibrillation efficacy.17) In addition, a prolonged duration of VF may raise the defibrillation thresholds.18) Special caution is required with these patients, and higher energy might result in greater efficiency. Prompt defibrillation is important to avoid repeated defibrillation and any deterioration of the hemodynamic status.
There were several limitations in this study. First, the low incidence of inadvertent VF is the major significant limitation. The incidence of inadvertent VF differs with EPS protocol and according to whether isoproterenol infusion or internal cardioversion is required to terminate arrhythmia.
Figures and Tables
Fig. 1
Patient No. 4 exhibited VF induction after incorrect R-wave synchronization of the direct current shock. Previous R-R interval before shock was 439 ms. QT interval of previous cardiac cycle and last QRS to shock interval were 204 and 177 ms, respectively. VF: ventricular fibrillation.
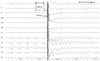
Fig. 2
Two AF patients (No. 5 and 8) exhibited spontaneous VF induction during isoproterenol infusion. Those two patients also received high-dose isoproterenol infusion (10 µg/min) to evaluate AF triggering spontaneous atrial foci. AF: atrial fibrillation, VF: ventricular fibrillation.
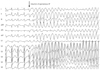
Fig. 3
Patient No. 2 developed VF after rapid atrial pacing with a cycle length of 210 ms in an attempt to induce AF. However, the catheter was accidentally moved to the RV apex. Surface ECG shows atrial capture at the beginning of the programmed stimulation that changed to ventricular capture when VF was induced. AF: atrial fibrillation, ECG: electrocardiogram, RV: right ventricular, VF: ventricular fibrillation.
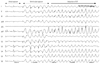
Fig. 4
A patient of VPB ablation (No. 1) spontaneously developed VF during infusion with low-dose isoproterenol at 3 µg/min. Soon after isoproterenol infusion, frequent polymorphic non-sustained VT was observed. This spontaneously degenerated into VF. VF: ventricular fibrillation, VPB: ventricular premature beat, VT: ventricular tachycardia.

References
1. Denes P, Ezri MD. Clinical electrophysiology--a decade of progress. J Am Coll Cardiol. 1983; 1:292–305.
2. Scheinman MM, Morady F. Invasive cardiac electrophysiologic testing: the current state of the art. Circulation. 1983; 67:1169–1173.
3. Surawicz B. Intracardiac extrastimulation studies: How to? Where? By whom? Circulation. 1982; 65:428–431.
4. Kanda M, Shimizu W, Matsuo K, et al. Electrophysiologic characteristics and implications of induced ventricular fibrillation in symptomatic patients with Brugada syndrome. J Am Coll Cardiol. 2002; 39:1799–1805.
5. Horowitz LN, Kay HR, Kutalek SP, et al. Risks and complications of clinical cardiac electrophysiologic studies: a prospective analysis of 1,000 consecutive patients. J Am Coll Cardiol. 1987; 9:1261–1268.
6. Murgatroyd FD, Slade AK, Sopher SM, Rowland E, Ward DE, Camm AJ. Efficacy and tolerability of transvenous low energy cardioversion of paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. 1995; 25:1347–1353.
7. Cooper RA, Alferness CA, Smith WM, Ideker RE. Internal cardioversion of atrial fibrillation in sheep. Circulation. 1993; 87:1673–1686.
8. Kettering K, Mewis C, Riemer M, Kühlkamp V. [Ventricular fibrillation in intra-atrial cardioversion of atrial fibrillation]. Z Kardiol. 2000; 89:269–273.
9. Barold HS, Wharton JM. Ventricular fibrillation resulting from synchronized internal atrial defibrillation in a patient with ventricular preexcitation. J Cardiovasc Electrophysiol. 1997; 8:436–440.
10. Sharma AD, Waxman MB, Huerta FI. Effect of adrenergic stimulation and blockade on ventricular defibrillation in the rat. J Pharmacol Exp Ther. 1983; 227:716–722.
11. Opie LH, Nathan D, Lubbe WF. Biochemical aspects of arrhythmogenesis and ventricular fibrillation. Am J Cardiol. 1979; 43:131–148.
12. Rosenthal RE, Turbiak TW. Open-chest cardiopulmonary resuscitation. Am J Emerg Med. 1986; 4:248–258.
13. Cohen TJ, Scheinman MM, Pullen BT, et al. Emergency intracardiac defibrillation for refractory ventricular fibrillation during routine electrophysiologic study. J Am Coll Cardiol. 1991; 18:1280–1284.
14. Babbs CF, Paris RL, Tacker WA Jr, Bourland JD. Effects of myocardial infarction on catheter defibrillation threshold. Med Instrum. 1983; 17:18–20.
15. Marinchak RA, Friehling TD, Kline RA, Stohler J, Kowey PR. Effect of antiarrhythmic drugs on defibrillation threshold: case report of an adverse effect of mexiletine and review of the literature. Pacing Clin Electrophysiol. 1988; 11:7–12.
16. Guarnieri T, Levine JH, Veltri EP, et al. Success of chronic defibrillation and the role of antiarrhythmic drugs with the automatic implantable cardioverter/defibrillator. Am J Cardiol. 1987; 60:1061–1064.
17. Lerman BB, Halperin HR, Tsitlik JE, Brin K, Clark CW, Deale OC. Relationship between canine transthoracic impedance and defibrillation threshold. Evidence for current-based defibrillation. J Clin Invest. 1987; 80:797–803.
18. Winkle RA, Mead RH, Ruder MA, Smith NA, Buch WS, Gaudiani VA. Effect of duration of ventricular fibrillation on defibrillation efficacy in humans. Circulation. 1990; 81:1477–1481.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download