Abstract
Background and Objectives
The intracoronary injection of acetylcholine (Ach) has been shown to induce coronary spasms in patients with variant angina. Clinical significance and angiographic characteristics of patients with a significant response to lower Ach dosages are as-yet non-clarified compared with patients responding to higher Ach doses.
Subjects and Methods
A total of 3034 consecutive patients underwent coronary angiography with Ach provocation tests from January 2004 to August 2010. Ach was injected in incremental doses of 20, 50, 100 µg into the left coronary artery. Significant coronary artery spasm was defined as focal or diffuse severe transient luminal narrowing (>70%) with/without chest pain or ST-T change on the electrocardiogram (ECG). We compared the clinical and angiographic characteristics of patients who responded to a lower Ach dose (20 or 50 µg, n=556) to those that responded to a higher Ach dose (100 µg, n=860).
Results
The baseline clinical and procedural characteristics are well balanced between the two groups, except diabetes was higher in the lower Ach dose group and there were differences in medication history. After adjusting for confounding factors, the lower Ach dose group showed more frequent temporary ST elevation and atrioventricular block on the ECG. Furthermore, the group of patients who responded to the lower Ach dose was associated with a higher incidence of baseline and severe spasm than those who responded to a higher Ach dose.
Conclusion
Patients with a significant response to a lower Ach dose were associated with more frequent ST elevation, baseline spasm, and more severe spasm compared with those who responded to a higher Ach dose, suggesting more intensive medical therapy with close clinical follow-up is required for those patients.
Endothelial dysfunction and subsequent coronary artery spasm (CAS) plays an important role in the pathogenesis of variant angina. The coronary arteries of patients with variant angina are hyperreactive to diverse constrictor stimuli, and occlusive constriction is readily induced by exposure to such a stimulus.1)2) In this situation, an intracoronary injection of acetylcholine (Ach) is useful for inducing significant CAS in patients with variant angina. The vascular effects of Ach on human coronary arteries are complex.3) In fact, Ach causes vasodilation, mediated by a release of endothelium-derived relaxing factors (EDRFs) in patients with preserved endothelial function, and vasoconstriction due to the direct stimulation of the vascular smooth muscle. The intracoronary infusion of Ach has reported to cause dilation of coronary arteries in patients without angiographic evidence of coronary atherosclerosis, but also causes a constriction of stenotic coronary arteries.4)5) Recently, instead of the intravenous administration of ergonovine, an intracoronary Ach provocation test has been employed in the clinical setting. A previous study reported that the clinical and angiographic characteristics of an Ach induced spasm was related to the dosage level of the intracoronary injection of Ach in the general population.6) However, the clinical significance and angiographic characteristics of patients with vasospastic angina, according to the stimulating Ach dose, remain to be clarified. In this study, we sought to clarify the clinical and angiographic characteristics according to different Ach doses in patients with vasospastic angina.
A total of 3034 consecutive patients (male 50.4%, mean age 54.6±12.4 years) who had typical or atypical chest pain underwent coronary angiography (CAG) at the cardiovascular center of Korea University Guro Hospital, Seoul, South Korea. Those patients who had less than a 30% fixed stenosis on quantitative coronary angiography (QCA) on diagnostic angiography underwent a subsequent intracoronary Ach provocation test, either via a transradial or transfemoral approach. Patients were excluded if they had one of the following conditions: prior coronary artery bypass graft, prior percutaneous coronary intervention, prior cerebrovascular disease, advanced heart failure (New York Heart Association class III or IV), or serum creatinine ≥3 mg/dL, because these conditions can be major causes of future adverse cardiovascular events and present a bias to CAS. Finally, a total of 1445 patients who had a positive provocation test to different stimulating Ach doses were entered for this analysis. Enrolled patients were divided into two groups according to two different Ach doses: the lower Ach dose group (positive provocation test to 20 µg or 50 µg, n=556 patients) and the higher Ach dose group (positive provocation test to 100 µg, n=860 patients). Clinical and angiographic characteristics during the Ach provocation test were then compared between the two groups.
The first investigations for suspected CAS included typical clinical history, non-invasive stress tests such as a treadmill test, ambulatory electrocardiogram (ECG), and stress echocardiography, with a subsequent invasive angiogram performed for confirmation. CAS was induced by the intracoronary injection of Ach after diagnostic angiography. Nitrates, calcium channel blockers (CCB), beta blockers, angiotensin converting enzyme inhibitors, and other vasodilators or vasoconstrictors were withheld at least 72 hours before CAG. Ach was injected over incremental doses of 20 (A1), 50 (A2), and 100 (A3) µg/min into the left coronary artery over a 1 minute period, with 5 minutes intervals, up to the maximum tolerated dose under continuous monitoring of an ECG and blood pressure measurement. Angiography was repeated after each Ach dose until a significant vasoconstriction (70%) response was obtained. Intracoronary infusion with 0.2 mg nitroglycerin (NTG) was administered after completing the Ach provocation test. Angiography was then performed 2 minutes later. If focal or diffuse significant vasoconstriction of coronary arteries was induced with any dose of Ach by visual assessment, any further Ach infusion was ceased. End systolic images for each segment of the left coronary artery were chosen according to the corresponding points on the electrocardiographic trace (QRS onset or end of T wave) and analyzed using the appropriate QCA system of the catheterization laboratory (FD-20, Phillips, Amsterdam, the Netherlands). The coronary artery diameters were measured with QCA before and after the administration of Ach at the site that showed the greatest changes following drug administration. Reference diameter was measured at the proximal and distal portion of each artery before and after the intracoronary Ach infusion, and the mean reference diameter was used to assess the extent of diameter narrowing. During the CAG and Ach provocation test, significant CAS was defined as focal or diffuse severe transient luminal narrowing (>70%) by visual assessment with or without chest pain, or ischemic ECG change such as ST-T segment elevation, depression (≥1 mm), or T wave inversion. Previously, we have published our data with Korean criteria in several international peer-reviewed journals.7)8) The normal coronary appearance was defined as less than 20% luminal narrowing on coronary angiogram, as measured with QCA. Myocardial bridge (MB) was considered when the characteristic phasic systolic compression of the coronary artery had a >30% decrease in diameter on the angiogram after intracoronary NTG infusion, usually in anterior-posterior cranial projection or right anterior oblique cranial projection. Multi-vessel spasm (MVS) was defined as significant CAS of ≥2 major epicardial coronary arteries. The presences of a baseline spasm was defined as focal or diffuse narrowing more than 30% in diameter on the angiogram before the Ach provocation test was applied, as compared with the final angiogram after intracoronary NTG infusion. Diffuse CAS was defined as significant CAS site length ≥30 mm.
Hypertension was defined as either systolic or diastolic elevation of blood pressure ≥140/90 mm Hg or ongoing antihypertensive pharmacological treatment. Dyslipidemia was defined as a total cholesterol level ≥200 mg/dL or current treatment with lipid-lowering drugs. Current smoking was defined as active smoking within the past 12 months. Diabetes mellitus was defined as a fasting blood glucose level ≥126 mg/dL, or any use of hypoglycemic agents or insulin. In the present study, if a patient's past history, medical records, present symptoms, or medical examination results accorded with one of the following criteria, the patients were diagnosed with peripheral artery disease: 1) Claudication symptoms with ankle brachial indices <0.90; 2) Claudication symptoms with findings of a significant lesion (≥70% diameter stenosis) in a peripheral artery by a doppler ultrasound, computed tomographic angiography, magnetic resonance angiography, or invasive angiography; 3) Symptomatic carotid, subclavian arterial disease (≥70% diameter stenosis) documented by image studies including computed tomographic angiography, magnetic resonance angiography, or invasive angiography. Insignificant coronary artery disease was defined as a ≤20% diameter stenosis in coronary arteries documented by image studies including computed tomographic angiography, magnetic resonance angiography, or invasive angiography.
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 17.0 (SPSS Inc., Chicago, IL, USA). For continuous variables, differences between the two groups were evaluated by an unpaired t-test or Mann-Whitney rank sum test. For discrete variables, differences were expressed as counts and percentages and analyzed with a χ2 or Fisher's exact test between the groups, as appropriate. Multivariate logistic regression analysis, which included baseline confounding factors, was used for assessing the independent predictors for significant CAS. A two-tailed p of <0.05 was considered to be statistically significant. Data were expressed as mean±standard deviations.
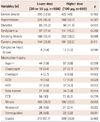
The baseline clinical characteristics of patients are illustrated in Table 1. Baseline clinical characteristics, including the prevalence of elderly status, hypertension, dyslipidemia, smoking history, chronic kidney disease, and congenital heart failure were balanced between the two groups. The prevalence of diabetes was higher in the lower Ach dose group. Before the Ach provocation test, antianginal medications such as nitrate, nicorandil, and trimetazidine were more frequently prescribed in the lower Ach dosage group.
The clinical and angiographic parameters during the Ach provocation tests are shown in Table 2. Before adjusting for baseline confounding factors, the incidences of baseline spasm (>30%) at the baseline coronary angiogram before Ach infusion was higher in the lower Ach dose group. The incidence of MB, one of the major determinants of significant CAS, was similar between the two groups. During the Ach provocation test, the incidences of ischemic ECG changes, particularly temporary ST elevation and atrioventricular (AV) blockage, were higher in the lower Ach dose group. QCA was performed in both groups and the QCA results are as shown in Table 2. Before the Ach provocation test, the reference vessel diameters before and after NTG infusion were similar between the two groups. After Ach infusion, mean % narrowing by QCA was found to be more severe in the lower Ach dose group. Furthermore, the lower Ach dose group was associated with a higher incidence of baseline spasm, severe vasospasm, MVS, and a trend toward a higher incidence of diffuse spasms (>30 mm) in the univariate analysis as compared with the higher Ach dose group. There was no difference between the two groups according to coronary artery location. However, after adjusting for baseline confounding factors, including gender, hypertension, diabetes, current smoking status, dyslipidemia, high sensitivity C-reactive protein, high density lipoprotein, and cholesterol, the lower Ach dose group was associated with more frequent baseline spasms and severe spasms.
The main findings of the present study are the following: Patients who demonstrate a significant response to a lower Ach dose are 1) more associated with ischemic ECG change, particularly transient ST elevation and AV blockage during the Ach provocation test, and 2) more associated with a higher incidence of baseline spasms and severe spasms, suggesting a higher degree of vulnerability and profound endothelial dysfunction to constrictor stimuli as compared with patients who show a significant response to a higher Ach dose. Therefore, those patients with CAS documented by lower Ach dose would require more aggressive and intensive medical therapy, including CCB, long-acting nitrate, and nicorandil. Close clinical follow-up would be required in real world clinical practice.
Recent reports have found showed the patients with MB had a higher incidence of Ach induced CAS than those of the control group (p<0.001).6) However, in the present study, because the incidence of MB was similar between the two groups, this factor had no impact on the clinical and angiographic characteristics of both groups during the Ach provocation test.
The role of CAS in the development of myocardial ischemia has been clarified.9) But its precise mechanism remains unclear. It is widely understood that the coronary arteries of patients with variant angina are hyperreactive against diverse constrictor stimuli,10) but it is controversial as to whether this abnormal constrictor response results from localized or diffuse vasomotility disorder in the coronary artery. The variability of the overall response to Ach as a function of the infused dose and of the coronary segment may be explained by local variations in the balance between its opposing vasomotor effects, indicating vasodilation caused by an endothelial release of EDRF and vasoconstriction caused by a direct stimulation of the vascular smooth muscle. It has been suggested that a constriction of atherosclerotic epicardial vessels in response to intracoronary Ach is paradoxical and indicative of widespread endothelial dysfunction.4)11) For evaluating the constrictor response of each left and right coronary artery separately, intracoronary Ach has the advantage over ergonovine of an extremely short half-life. Therefore, if spasm is induced, it resolves spontaneously in many patients, whereas ergonovine can be associated with delayed spasm and a possible subsequent coronary event.
It is possible to evaluate the dose-dependent effect of Ach on each of the coronary arteries during the attempt to induce CAS. Because a spasm of both coronary arteries does not occur simultaneously using this method, even among patients with multi-vessel CAS, hemodynamic instability rarely develops. Thus, the coronary angiogram of each coronary artery can be obtained during a spasm in a safe and timely fashion.12)
Although bradyarrhythmias, including sinus bradycardia and AV blockage, may occur after the injection of Ach, particularly into the right coronary artery, these bradycardias are transient and easily controlled by an induced cough or temporary ventricular pacing.13) A previous study showed that CAS can be induced by the intracoronary injection of Ach, a parasympathetic neurotransmitter, and the spasm can be suppressed by atropine, a parasympatholytic agent the sensitivity of which has been found to be related to the activation of the autonomic nervous system.14)
In the present study, the lower Ach dose group showed more frequent ischemic ECG changes, including ST-segment elevation and AV blockage, during the Ach provocation test, suggesting worse angiographic characteristics may have induced worse clinical symptoms and signs during the Ach provocation test. The lower Ach dose group also showed a higher incidence of baseline spasm before Ach infusion, showing spasticity and a vulnerable trend of coronary arteries to constrictor stimuli. After Ach infusion, mean % narrowing and minimal luminal diameter (mm) by QCA were more severe in the lower Ach dose group. Therefore, the patients with a lower Ach dose are very sensitive and vulnerable to Ach stimuli as compared with patients administered higher Ach doses. Thus, the lower Ach dose group should be managed with more aggressive medical treatment, with antianginal agents, and would require close clinical observation.
It is of interest that patients in the lower Ach dose group in whom severe CAS was demonstrated were administered more antianginal mediations, such as nitrate (29.3%), nicorandil (4.6%), and trimetazine (4.6%), than those in the higher Ach dose group, empirically, based on the outpatient clinic records. Lee et al.15) reported an increased basal tone, hyperresponsiveness to Ach, and ergonovine in spasm-related coronary arteries in patients with variant angina. In our study, the lower Ach dose group showed more baseline spasms (36.6%) than the higher Ach dose group, suggesting that basal coronary artery tone is increased in patients with variant angina with higher disease activity in the spasm-related artery. This may be related to the occurrence of CAS. The level of generalized vasoconstrictive stimuli may determine the basal coronary artery tone as well as the sensitivity to Ach or ergonovine at the spastic segments in patients with variant angina.16)17) According to these findings, the basal coronary artery tone is elevated by enhanced vasoconstrictive stimuli, and the CAS may result from locally exaggerated responses to the vasoconstrictive stimuli of the spasm-related arteries, in which endothelial dysfunction and superimposed local hyperreactivity of the vascular smooth muscles may be present.
In this context, we tested the hypothesis that the lower Ach dose group may suffer from more diffuse, severe, and MVSs than the higher Ach dose group in patients with vasospastic angina. However, after adjustment for confounding factors, we were only able to observe that the lower Ach dose group was associated with a higher incidence of baseline and severe spasms. Of course, some earlier studies6)18)19) have indicated that the clinical and angiographic characteristic of Ach induced spasm was related specifically to the dosage of intracoronary injection of Ach in the general population.
This study has several obvious limitations. First, in the guidelines for diagnosis and treatment of patients with vasospastic angina (Japanese Coronary Association 2008), CAS is defined as the "transient, total, or sub-total occlusion (>90% stenosis) of a coronary artery with a sign/symptoms of myocardial ischemia (anginal pain and ischemic ST changes).20) However, we considered patient safety and convenience in real world practice, thus, we did not wait until subtotal or total occlusion through maximal Ach stimuli. We considered safety to be more important when performing an Ach provocation test on the outpatient clinic base. Therefore, in our study, significant CAS was defined as focal or diffuse severe transient luminal narrowing (>70%) with or without chest pain, or ischemic ECG change, such as ST-T segment elevation, depression (≥1 mm), or T wave inversion. This might have resulted in a significant risk of false (+) findings, if >70% diffuse coronary spasm is regarded as a (+) result due to differences of diagnostic criteria. Second, the limitations of our study include the observational retrospective design, which lacks randomization and may inadvertently introduce a selection bias. Third, even though we minimized the confounding effects from the baseline biases with multivariate logistic analysis, it is possible that some potential confounders may have impacted on our results. However, we tested CAS in a relatively larger study population, which is helpful in minimizing any confounding effects from the baseline biases. Fourth, the Ach provocation test was not performed in the early morning. It was usually performed around noon. The circadian variation of coronary spasms may21) be the reason why the positive provocation rate for CAS was higher in our study than that observed in previous reporting.22) Fifth, because we enrolled all patients who had chest pain (without concern about typical or atypical chest pain), without significant coronary artery stenosis, the typical expected (+) rate of spasm may have been higher in this study. Therefore, we enrolled only >70% diffuse coronary spasm patients, as this is not currently regarded as variant angina.
The sixth limitation is the study population, in which there may have been present a bias toward those who had undergone the Ach provocation tests. Ach provocation tests were performed to document significant CAS for the diagnosis of variant angina. Thus, this study was not designed to evaluate the degree of endothelial dysfunction in patients with variant angina.
In conclusions, the present study found that patients with vasospastic angina documented by a lower Ach dose provocation test have a higher incidence of transient ST elevation, baseline spasm, and more severe spasms than patients who responded to a higher dose of Ach. Therefore, in the real clinical world, patients with significant CAS documented by a lower Ach dose would require more aggressive and intensive medical therapy and careful clinical follow-up would be required for this particular subset of patients.
Figures and Tables
References
1. Nardi F, Verna E, Secco GG, et al. Variant angina associated with coronary artery endothelial dysfunction and myocardial bridge: a case report and review of the literature. Intern Med. 2011; 50:2601–2606.
2. Kawano H, Ogawa H. Endothelial function and coronary spastic angina. Intern Med. 2005; 44:91–99.
3. el-Tamimi H, Davies GJ, Crea F, Maseri A. Response of human coronary arteries to acetylcholine after injury by coronary angioplasty. J Am Coll Cardiol. 1993; 21:1152–1157.
4. Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986; 315:1046–1051.
5. Horio Y, Yasue H, Rokutanda M, et al. Effects of intracoronary injection of acetylcholine on coronary arterial diameter. Am J Cardiol. 1986; 57:984–989.
6. Sueda S, Kohno H, Fukuda H, et al. Clinical and angiographical characteristics of acetylcholine- induced spasm: relationship to dose of intracoronary injection of acetylcholine. Coron Artery Dis. 2002; 13:231–236.
7. Chen KY, Rha SW, Li YJ, et al. Impact of hypertension on coronary artery spasm as assessed with intracoronary acetylcholine provocation test. J Hum Hypertens. 2010; 24:77–85.
8. Im SI, Rha SW, Choi BG, et al. Angiographic and Clinical Characteristics according to Intracoronary Acetylcholine Dose in Patients with Myocardial Bridge. Cardiology. 2013; 125:250–257.
9. Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011; 124:1774–1782.
10. Kaski JC, Crea F, Meran D, et al. Local coronary supersensitivity to diverse vasoconstrictive stimuli in patients with variant angina. Circulation. 1986; 74:1255–1265.
11. Newman CM, Maseri A, Hackett DR, el-Tamimi HM, Davies GJ. Response of angiographically normal and atherosclerotic left anterior descending coronary arteries to acetylcholine. Am J Cardiol. 1990; 66:1070–1076.
12. Yasue H, Horio Y, Nakamura N, et al. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986; 74:955–963.
13. Okumura K, Yasue H, Horio Y, et al. Multivessel coronary spasm in patients with variant angina: a study with intracoronary injection of acetylcholine. Circulation. 1988; 77:535–542.
14. Sakata K, Miura F, Sugino H, et al. Assessment of regional sympathetic nerve activity in vasospastic angina: analysis of iodine 123-labeled metaiodobenzylguanidine scintigraphy. Am Heart J. 1997; 133:484–489.
15. Lee SJ, Park SJ, Park SW, et al. Increased basal tone and hyperresponsiveness to acetylcholine and ergonovine in spasm-related coronary arteries in patients with variant angina. Int J Cardiol. 1996; 55:117–126.
16. Kaski JC, Maseri A, Vejar M, Crea F, Hackett D. Spontaneous coronary artery spasm in variant angina is caused by a local hyperreactivity to a generalized constrictor stimulus. J Am Coll Cardiol. 1989; 14:1456–1463.
17. Kuga T, Egashira K, Inou T, Takeshita A. Correlation of basal coronary artery tone with constrictive response to ergonovine in patients with variant angina. J Am Coll Cardiol. 1993; 22:144–150.
18. Okumura K, Yasue H, Matsuyama K, et al. Diffuse disorder of coronary artery vasomotility in patients with coronary spastic angina. Hyperreactivity to the constrictor effects of acetylcholine and the dilator effects of nitroglycerin. J Am Coll Cardiol. 1996; 27:45–52.
19. Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: The CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) Study. J Am Coll Cardiol. 2008; 52:523–527.
20. JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J. 2010; 74:1745–1762.
21. Yasue H, Omote S, Takizawa A, Nagao M, Miwa K, Tanaka S. Circadian variation of exercise capacity in patients with Prinzmetal's variant angina: role of exercise-induced coronary arterial spasm. Circulation. 1979; 59:938–948.
22. Okumura K, Yasue H, Matsuyama K, et al. Sensitivity and specificity of intracoronary injection of acetylcholine for the induction of coronary artery spasm. J Am Coll Cardiol. 1988; 12:883–888.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download