Abstract
Atrial fibrillation (AF) is the most common chronic arrhythmia in the world, and it is associated with an increased long-term risk of stroke, heart failure, and all-cause mortality. To overcome the limitations of transvenous radiofrequency (RF) ablation for AF, total thoracoscopic ablation (TTA) has evolved as a new technique. TTA has several advantages over transvenous RF ablation and is known to produce better outcomes, especially in patients with persistent AF. Herein, we report 2 cases of successful TTA followed by an electrophysiological study confirming satisfactory ablation lines; the first such procedure reported in Korea.
Atrial fibrillation (AF) is the most common chronic arrhythmia in the world, with an estimated prevalence of 0.4-1% in the general population.1) AF is associated with an increased long-term risk of stroke, heart failure, and all-cause mortality.2) Although the Cox-maze III procedure is an effective treatment, the procedure has failed to achieve widespread adoption because it is highly invasive.
Haissaguerre et al. identified the pulmonary veins (PV) as potential sources of AF, and this led to the development of transvenous radiofrequency (RF) ablation of the PV. Although transvenous RF ablation eliminates AF in a high proportion of cases, PV reconduction and AF recurrence is problematic, most likely due to the difficulties in achieving transmural lesions by this technique.3) Alternative approaches, such as thoracoscopic epicardial PV isolation, have evolved to overcome the difficulties with RF ablation. At the current time, RF ablation has evolved into an epicardial bipolar RF ablation of the PV, detection and ablation of ganglionated plexi (GP), division of the ligament of Marshall, and removal of the left atrial appendage (LAA). Recent reports have shown that this technique results in AF elimination in 80-91% of patients.4) More recently, some authors have suggested using a hybrid surgical-electrophysiological (EP) approach to confirm ablation lines because of the relationship between recurrences of AF or atrial tachycardia and conduction across ablation lines.5)
Herein, we describe the first experiences with total thoracoscopic ablation (TTA) followed by an EP study for longstanding persistent AF in Korea.
We explained all about TTA to AF patients who were interested in this new technique before surgery because TTA can be performed in patients with both persistent and paroxysmal AF. For the first time in our institution, 2 patients with persistent AF were treated with TTA. Both patients wanted to quit warfarin in spite of symptom improvement using antiarrhythmic drugs and wanted to undergo TTA on a voluntary basis. The first patient was a 66-year-old man with a 12-month history of persistent AF. His chief complaints were palpitations and dizziness. His medications included warfarin and sotalol. One attempt of cardioversion did not work. Coronary angiography and echocardiography showed that he had normal coronary arteries, normal heart valve function, left atrial diameter was 38 mm, left atrial volume index was 54.9 mL/m2, left ventricular ejection fraction was 60%, and no intracardiac thrombi or vegetations were seen by transesophageal echocardiography. The second patient was 50-year-old man with a 5-year history of persistent AF. His chief complaints were dyspnea on exertion and palpitations; in addition, this patient had on-going problems with hypertension, diabetes, and alcoholism. His medications included warfarin and bisoprolol. Two attempts at cardioversion failed. Echocardiography showed that his left ventricular and heart valve function were normal and there were no intracardiac thombi, but the left atrial diameter was increased to 60 mm and left atrial volume index was 66 mL/m2.
Both patients were admitted 3 days prior to their respective surgery. Oral anticoagulation was transitioned to IV heparin, transesophageal echocardiography was performed to exclude left atrial thrombi, and antiarrhythmic drugs were continued during the hospital stay. Other preoperative evaluations included a 12-lead electrocardiography, chest radiography, pulmonary function testing, coronary computed tomography, and laboratory tests.
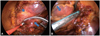
Both TTAs were performed through 3 ports at both sides under general anesthesia with double-lumen endotracheal intubation for selective pulmonary ventilation. Starting on the right side, a 5 mm port was introduced in the fourth intercostal space at the mid-axillary line. Carbon dioxide insufflation was used to expand the operative field and depress the diaphragm. Using a 0-degree scope, good visualization was obtained. The remaining 2 ports were placed in the third intercostal space at the anterior axillary line and the sixth intercostal intercostal space at the mid-axillary line. After pericardial tenting and blunt dissection of the Waterstone groove, a lighted dissector (AtriCure Lumitip Dissector, Atricure, Inc., Cincinnati, OH, USA) was used to pass a rubber band under the PV antrum. Then, an AtriCure Isolator Transpolar Clamp (Atricure, Inc., Cincinnati, OH, USA) (Fig. 1A) was connected to the rubber band and positioned around the PV antrum. PV antrum isolation was performed via the application of bipolar RF energy 6 times to the clamps around the PV antrum. To prevent macro-reentry, we made an additional inferior ablation line connecting both PV isolation lines. GPs were subsequently ablated with bipolar RF energy. GPs around PV antrum were ablated with AtriCure Cooltip pen (Atricure, Inc., Cincinnati, OH, USA) three times. Confirmation of ablation lines was performed via pacing testing using the AtriCure Cooltip pen. The procedure was then repeated on the left side. A 5 mm port was introduced in the left fourth intercostal space at the mid-axillary line for the thoracoscope. The locations of the remaining 2 ports were symmetrical bilaterally. After PV and GP ablation, the ligament of Marshall was dissected and ablated. After all ablations were completed and conduction block was confirmed, the LAA was removed with an endoscopic stapling device (Fig. 1B). Single chest tubes were inserted bilaterally. The patients were monitored for the first 24 hours in the intensive care unit. Postoperatively, both patients received warfarin and amiodarone for 6 months. During follow-up, we had each patient undergo 24 hours of Holter monitoring at 6 months postoperative; at one-year postoperative, both patients will undergo 2-weeks long-term monitoring.
The right and left groin areas and left subclavian area were infiltrated with lidocaine. Sheaths were placed: one 7.5 Fr sheath and one 6 Fr sheath in the right femoral vein. A quadripolar catheter was placed from the right femoral vein into the apex of the right ventricle. After positioning the catheter, one SL1 sheath was introduced into the left atrium through transseptal puncture using fluoroscopy and blood pressure monitoring. Systemic anticoagulation with intravenous bolus injection of 3000 U heparin was initiated just after the trans-septal insertion of the sheath. Under fluoroscopic exam, the SL1 sheath position was confirmed. We inserted lasso catheters of 15-25 mm diameter into the PVs and their potentials were checked. We measured the conduction intervals of posteroanterior, AH, and. We determined antegrade and retrograde refractory periods. After the procedures were completed, we pulled out all catheters and a complete hemostasis was performed on each patient.
Sinus rhythm was restored via cardioversion just after TTA. Other than 3 transient episodes of AF during his remaining hospitalization, the patient maintained a sinus rhythm. The patient was in the condition for discharge at postoperative fourth day but he waited for staged EP study. This patient remained in hospital for 8 days. An EP study on the sixth day postoperative showed "successful antral ablation in all PV". At 6-month follow-up, the patient was in sinus rhythm on 24 hours Holter monitoring. The anticoagulation therapy was stopped.
Sinus rhythm was restored immediately after right PV ablation. During hospitalization, there were no episodes of AF recurrence. However, the patient's pulse was slightly increased at 100, and metoprolol was prescribed for rate control. The hospital stay was 8 days. The staged EP study was performed on the fourth day postoperative. The postoperative EP study showed "successful antral ablation in all PV except a small potential in the left PV". The small potential was thought to be a signal far-field; therefore, additional management was not indicated. This small potential could not be found at follow-up EP study 3 months later. At 6-months' follow-up, the patient was in sinus rhythm with 3 atrial premature complexes on 24 Holter monitoring. The anticoagulation therapy was stopped.
In this report, we describe our first experiences with TTA; this included PV isolation, GP ablation, LAA removal, ablation of the posterior left atrium, and the division of the ligament of Marshall. In addition, we performed subsequent EP mapping as part of our hybrid approach.
In the literature there are various reports of minimally invasive or thoracoscopic ablation of the PV.6-8) Most of these reports emphasize GP ablation and LAA removal as advantages over transvenous RF ablation. However, whether GP ablation improves the success rate of minimally invasive ablation compared with surgical PV isolation is unknown. One retrospective study reported that vagal denervation enhanced the long-term benefit of circumferential ablation for treatment of paroxysmal AF.9) On the other hand, Oh et al.10) reported that epicardial fat pad ablation, comparable to GP ablation, did not suppress AF induction. In our first experiences, we have performed high-frequency stimulation (300 pulses per minute) around the PV antrum to confirm the complete ablation of GPs around PV antrum. A positive response was defined as bradycardia with a doubling of the R-R interval; this was achieved in both patients after PV ablation. Additional GP ablation was performed and neither patient experienced recurrence of their persistent AF after 2 months of follow-up. Based on this finding, GP ablation might be helpful in maintaining a sinus rhythm, although we acknowledge that the longterm effects of GP ablation are unknown. Further studies are needed to elucidate the necessity and effects of GP ablation.
Left atrial appendage excision lowers thromboembolic risk independent of the cardiac rhythm.11) This is important because many patients have a strong desire to stop warfarin therapy. Edgerton et al.,8) a leading group in TTA, were able to stop anticoagulation therapy in nearly 82% of patients that had no LAA and were free of AF 6 months after TTA, with no postoperative transient ischemic attacks or cerebrovascular accidents. We adopted their postoperative strategy of removing the LAA and warfarin anticoagulation for 6 months; neither patient had a thromboembolic event during follow-up.
The collaboration between surgeons and cardiologists is beneficial to procedural outcomes. In the patient who had a large left atrium (60 mm), small residual potentials in the left PV were found on the EP study despite intraoperative confirmation of the ablation lines. These potentials were small enough that additional management was not indicated, but they may cause AF recurrence in the longterm. Based on this experience, we recommend consideration of a hybrid procedure for TTA and an EP study, including possible RF ablation, in patients with a large atrium, which is a risk factor for AF recurrence.
In summary, we performed the first successful TTA of the PV in Korea with satisfactory follow-up EP mapping. Moreover, GP ablation, division of the ligament of Marshall, removal of the LAA and additional ablation lines, such as a posterior line, were made without difficulties. These results show that a hybrid procedure should be considered in patients with a high risk of AF recurrence.
Figures and Tables
References
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370–2375.
2. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year followup of the Renfrew/Paisley study. Am J Med. 2002; 113:359–364.
3. Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg. 2005; 130:797–802.
4. Sagbas E, Akpinar B, Sanisoglu I, et al. Video-assisted bilateral epicardial pulmonary vein isolation for the treatment of lone atrial fibrillation. Ann Thorac Surg. 2007; 83:1724–1730.
5. Jansen WPJ, Wijffels MC, Wever EFD, van Boven WJ, Yilmaz A, Boersma LV. Recurrence of atrial fibrillation after mini-Maze is associated with pulmonary vein reconnection. Eur Heart J. 2009; 30:Suppl 1. 813.
6. Edgerton JR, Jackman WM, Mack MJ. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. J Interv Card Electrophysiol. 2007; 20:89–93.
7. McClelland JH, Duke D, Reddy R. Preliminary results of a limited thoracotomy: new approach to treat atrial fibrillation. J Cardiovasc Electrophysiol. 2007; 18:1289–1295.
8. Edgerton JR, Edgerton ZJ, Weaver T, et al. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. Ann Thorac Surg. 2008; 86:35–38. discussion 39.
9. Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004; 109:327–334.
10. Oh S, Zhang Y, Bibevski S, Marrouche NF, Natale A, Mazgalev TN. Vagal denervation and atrial fibrillation inducibility: epicardial fat pad ablation does not have long-term effects. Heart Rhythm. 2006; 3:701–708.
11. Halperin JL, Hart RG. Atrial fibrillation and stroke: new ideas, persisting dilemmas. Stroke. 1988; 19:937–941.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download