Abstract
Endovascular aneurysm repair (EVAR) using stent grafts has shown to be an effective alternative to surgical repair in treating an abdominal aortic aneurysm (AAA). EVAR is associated with shorter hospital stays, less blood loss, shorter operating times, and lower early morbidity and mortality compared to open surgical repair, although EVAR required a higher reintervention rate during a longer follow-up period. However, short or severely an angulated infrarenal proximal aortic neck is considered unsuitable for EVAR. The chimney graft technique is a modified procedure based on the deployment of a covered or bare-metal stent parallel to the main aortic endograft within the aneurysm, thereby creating a conduit that runs outside the aortic main endograft to preserve flow to the aortic branches. In this case report, we present a 78-year-old patient with an AAA with a short and severely angulated proximal neck who was successfully treated by EVAR using the chimney graft technique.
Endovascular aneurysm repair (EVAR) with stent grafts have revolutionized the treatment of abdominal aortic aneurysms (AAA) and have now become the procedure of choice especially in patients deemed intermediate or high risk for open aneurysm repair.1-3) Referral-based series from individual centers of excellence describe a 30-day perioperative mortality of 1 to 5% following elective open infrarenal AAA repair.4)5) However, the feasibility of EVAR is dependent on specific anatomical criteria. AAA with a short (<15 mm) or angulated (>60°) proximal neck is generally considered unsuitable for EVAR. Consequently, a complex open surgical repair with higher risk of mortality and morbidity remains the preferred treatment option in patients with unfavorable proximal neck anatomy.
After the chimney graft technique was introduced, it drastically expanded EVAR's applicability as a treatment option for AAA. Patients who were not previously considered candidates for EVAR were subject to open surgical repairs based on previous guidelines and now have another treatment option.
In this case report, we describe a 78-year-old patient with an AAA with a short and severely angulated proximal neck who was successfully treated by EVAR using the chimney graft technique.
A 78-year-old male with a known infrarenal AAA presented with recent left flank pain at the Urology Department of Severance Hospital. The patient had previously undergone a percutaneous transluminal coronary angioplasty at the mid right coronary artery (RCA) due to inferior wall myocardial infarction 22 years ago and had been regularly followed up. In addition, he also had a past history of hypercholesterolemia and benign prostate hyperplasia. He reported being a non-smoker without hypertension or diabetes. The AAA was incidentally discovered on coronary computed tomography (CT) taken six years ago and had a maximal diameter of 33 mm.
The CT taken prior to admission demonstrated a thrombosed infrarenal aortic aneurysm with an increased maximum diameter of 63 mm, which prompted a referral to Cardiology (Fig. 1). The proximal aortic neck had an angulation of approximately 45° to the suprarenal aorta, and the length of the aortic neck to the right renal artery orifice was 16 mm. However, the left renal artery orifice was adjacent to the aneurysm, and there was practically no neck. Therefore, if the conventional EVAR technique were to be used, the blood flow to the left renal artery would be compromised due to the stent, inevitably resulting in decreased renal function.
Based on the patient's advancing age and history of myocardial infarction, a preprocedural coronary evaluation was performed. An electrocardiogram showed normal sinus rhythm with ischemic changes in inferior leads, and echocardiography showed reduced left ventricular systolic function with an ejection fraction of 48% and akinesia of the RCA territory. The coronary angiography showed significant tandem stenotic lesions of the proximal and distal left anterior descending artery, which were subsequently treated with drug-eluting stents (Endeavor Sprint 3.0×24 mm and 2.5×18 mm, Medtronic, Santa Rosa, CA, USA).
Considering the patient's age and history of significant coronary artery disease, we decided to perform EVAR using the chimney technique for AAA during his hospital stay.
Vascular access in both femoral arteries was obtained percutaneously and was preclosed using two suture type closure devices (ProGlide, Abbott, Abbott Park, IL, USA) on each side. A 7 Fr Shuttle Sheath was inserted into the suprarenal aorta through the left brachial artery access. The left renal artery was cannulated using a 5 Fr Multi-purpose catheter through the Shuttle Sheath. A 0.035" wire (Amplatz stiff wire Cook Medical Inc., Bloomington, IN, USA) was inserted into the left renal artery, and a 6×50 mm graft stent (Viabahn, Gore, Flagstaff, AZ, USA) was placed into the left renal artery along the wire.
A 36×70 mm aortic extension stent graft (Endurant, Medtronic) was inserted into the abdominal aorta through the right femoral artery and deployed so that the upper end of the graft of the aortic extension is positioned just below the right renal artery. The ostium of the left renal artery was covered by the graft (Fig. 2A). Subsequently, the Viabahn stent graft (Gore Viabahn Endoprothesis, W.L. Gore and Associates, Flagstaff, AZ, USA) placed in the left renal artery was deployed. Thereafter, a 36-20-166 mm bifurcated Endurant main body stent was inserted into the abdominal aorta through the left femoral artery and deployed in overlap with the aortic extension (Fig. 2B). Also, a 16-24-93 mm contralateral limb piece (Endurant, Medtronic) was inserted through the right femoral artery and deployed.
The apposition of the aortic extension and the Viabahn stent graft to the aortic wall was achieved by a kissing balloon method using a 46 mm Reliant balloon and a 6×40 mm peripheral angioplasty balloon (Fig. 2C). Immediate post-procedure angiography showed no significant endoleak and patent left renal artery (Fig. 3). The left brachial artery access site was manually compressed and the femoral puncture sites were closed by tightening the prepared sutures.
No endoleaks or other complications were noted on routine postprocedural follow-up CT (Fig. 4). The laboratory results showed the patient's creatinine level at 1.14 mg/dL, which had no interval change of creatinine (baseline creatine 1.2 to 1.4 mg/dL) and estimated glomerular filtration rate.
The patient developed a fever after the procedure, but with no evident leukocytosis and negative blood culture, it was considered as a post-implant syndrome. The patient was discharged without other significant complications.
Randomized trials have demonstrated that EVAR has shorter hospital stays, less blood loss, shorter operating times, and lower perioperative mortality and morbidity compared to those of open surgical repair.1)6) Longer-term outcomes also seem to further favor of endovascular repairs, although more interventions were required after in EVAR over a longer follow-up.1)6)7) However, the anatomical suitability of AAA restricts the general applicability of EVAR. Anatomic suitability is generally defined as the absence of the following characteristics:
1) proximal aneurysm neck length less than 15 mm, 2) proximal neck diameter greater than 28 mm, 3) proximal neck angulation greater than 60 degrees in one or more planes, 4) extreme aortic or iliac tortuosity, defined in most cases as the presence of two or more 90-degree angulations within an iliac artery or subjectively as the inability to deliver a device without adjunctive measures, including modified access procedures or delivery adjuncts such as brachiofemoral access, 5) concomitant occlusive disease with an external or common iliac artery diameter less than 8 mm, and 6) inability to preserve at least one hypogastric artery by means of standard endovascular techniques.8)9)
Of these, the adverse anatomy of the infrarenal aneurysm neck is the predominant reason patients are excluded from EVAR candidacy. Although the threshold of 10 mm has been stipulated as sufficient to produce an adequate sealing zone for stent-graft positioning in more recent studies,7)9) the risk of type I proximal endoleak increases significantly with decreasing infrarenal neck length.10) The chimney graft technique, originally developed as a salvage procedure for saving visceral arteries that accidentally get covered during EVAR, can be a solution to this problem.
The chimney graft technique creates a conduit that runs outside the aortic main endograft by deploying a covered or bare-metal stent parallel to the aortic endograft, and has been proposed to ensure secure proximal fixation extending the sealing zones.11) Recent studies report that this technique can be an alternative treatment option to replace the complex open surgical repair of AAAs in high-risk patients with very short infrarenal necks.11)12) The chimney technique also can be an alternative to the fenestrated and branched stent-graft for preserving blood flow to side branches in the sealing zones of aortic stent-grafts.13) Fenestrated endovascular grafts (f-EVAR) require high-resolution imaging studies and the implantation procedure is challenging for the operator. Although recent improvements of fenestrated grafts have demonstrated promising results, the use of such devices mandates highly precise planning, a manufacturing delay of 6 to 12 weeks, and an expensive cost for manufacturing as the devices are customized for each patient's anatomy.14) However, the chimney graft is a simple technique that can be accomplished with regular stents and stent grafts and less radiation exposure compared with f-EVAR, especially beneficial in emergency settings.15)
The main question against the efficacy of the chimney graft technique lies in the development of type I endoleak, mainly due to the inadequate proximal sealing of the repair. Proximal fixation forces of the graft could be decreased due to modification of the graft-aorta interface, because covered stents placed between the endograft and the aortic wall decreases the extent of the contact surface area between the endograft and the aorta.
The reported incidence of type I endoleak after EVAR using the chimney technique is variable, ranging from 0 to 37.5%.16) In a recent article review reported by Moulakakis et al.,12) in which a total of 93 patients were analyzed, type I endoleak was present in 10.7% (10/93) of patients postoperatively, excluding three endoleaks that were detected and treated intraoperatively.
With the chimney graft technique, type I endoleak is usually of low flow because the gutters between the main aortic graft, the chimney graft, and the aortic wall was created as narrow as possible during the procedure. Thus, the accurate estimation of the diameter for the native aorta and the appropriate diameter of the deployed grafts may be of great importance for the prevention of type I endoleaks when using the chimney technique.
Another concern related to the chimney technique is the potential for procedure-related renal function impairment. In a recent review study, postprocedural renal function was impaired in 11.8% of the patients.12) Whether a hyperplastic response to the renal stent will ultimately threaten renal perfusion needs to be investigated. A thorough follow-up of renal function using a laboratory study is needed.
Late patency and the stability of chimney grafts are unknown. Thus, long-term follow-up studies are required to assess the efficacy and safety of the chimney technique.
In summary, an elderly AAA patient with a short angulated proximal neck was treated successfully with EVAR using the chimney technique. The use of the chimney technique expands the applicability of EVAR for the management of complex AAAs. However, the efficacy and safety of this endovascular technique needs to be investigated in further long-term studies.
Figures and Tables
Fig. 1
Baseline CT images of abdominal aortic aneurysm. A: three dimensional reconstruction image of the abdominal aortic aneurysm. B: axial view of abdominal aortic aneurysm at right renal artery (upper) and left renal artery level (middle) with a maximum diameter of 63 mm (below). C: coronal view of the infrarenal abdominal aortic artery, the black arrow indicates the length of the aortic neck to the right renal artery orifice which was 16 mm, and the white arrow indicates the left renal artery. The left renal artery orifice was adjacent to the aneurysm and there was practically no neck.
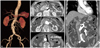
Fig. 2
Procedure steps of the chimney technique. A: insertion of an aortic extension stent graft (36×70 mm, Endurant, Medtronic, Santa Rosa, CA, USA) and a renal stent graft (6×50 mm, Viabahn, Gore, Flagshiff, AR, USA). B: the main body (36×20×166 mm, Endurant, Medtronic) was inserted at AAA in overlapping with the distal part of the aortic extension stent graft. C: adjuvant balloon dilation was performed by kissing balloon technique using a 46 mm Reliant balloon (Medtronic) for aorta stent graft and a 6×40 mm balloon for the left renal stent graft. D: additional balloon dilation of bifurcated stent graft was performed.
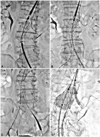
Fig. 3
Angiographic result after endovascular aneurysm repair using the Chimney graft technique. A: final angiogram showing no significant endoleak and patent left renal artery. B: stent at main body and left artery. C: flow through the left renal artery.

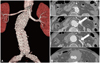
Fig. 4
A CT scan taken 4 days after the procedure showing complete exclusion of the abdominal aortic aneurysm sac and patent Viabahn stent graft deployed in the left renal artery. A: 3 dimensional reconstruction view. B: axial view of the aortic stent. White arrows indicate the stent graft in the left renal artery.
References
1. Schermerhorn ML, O'Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008; 358:464–474.
2. Brewster DC, Cronenwett JL, Hallett JW Jr, et al. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003; 37:1106–1117.
3. Lindsay TF. Canadian Society for Vascular Surgery. Canadian Society for Vascular Surgery consensus statement on endovascular aneurysm repair. CMAJ. 2005; 172:867–868.
4. Ernst CB. Abdominal aortic aneurysm. N Engl J Med. 1993; 328:1167–1172.
5. Hertzer NR, Mascha EJ, Karafa MT, O'Hara PJ, Krajewski LP, Beven EG. Open infrarenal abdominal aortic aneurysm repair: the Cleveland Clinic experience from 1989 to 1998. J Vasc Surg. 2002; 35:1145–1154.
6. EVAR trial participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005; 365:2187–2192.
7. Greenberg R, Fairman R, Srivastava S, Criado F, Green R. Endovascular grafting in patients with short proximal necks: an analysis of short-term results. Cardiovasc Surg. 2000; 8:350–354.
8. Greenberg RK, Clair D, Srivastava S, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003; 38:990–996.
9. Stanley BM, Semmens JB, Mai Q, et al. Evaluation of patient selection guidelines for endoluminal AAA repair with the Zenith Stent-Graft: the Australasian experience. J Endovasc Ther. 2001; 8:457–464.
10. Leurs LJ, Kievit J, Dagnelie PC, Nelemans PJ, Buth J. EUROSTAR Collaborators. Influence of infrarenal neck length on outcome of endovascular abdominal aortic aneurysm repair. J Endovasc Ther. 2006; 13:640–648.
11. Donas KP, Pecoraro F, Torsello G, et al. Use of covered chimney stents for pararenal aortic pathologies is safe and feasible with excellent patency and low incidence of endoleaks. J Vasc Surg. 2012; 55:659–665.
12. Moulakakis KG, Mylonas SN, Avgerinos E, et al. The chimney graft technique for preserving visceral vessels during endovascular treatment of aortic pathologies. J Vasc Surg. 2012; 55:1497–1503.
13. Ohrlander T, Sonesson B, Ivancev K, Resch T, Dias N, Malina M. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther. 2008; 15:427–432.
14. Greenberg RK, Sternbergh WC 3rd, Makaroun M, et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2009; 50:730–737.e1.
15. Panuccio G, Greenberg RK, Wunderle K, Mastracci TM, Eagleton MG, Davros W. Comparison of indirect radiation dose estimates with directly measured radiation dose for patients and operators during complex endovascular procedures. J Vasc Surg. 2011; 53:885–894.e1. discussion 894.
16. Hiramoto JS, Chang CK, Reilly LM, Schneider DB, Rapp JH, Chuter TA. Outcome of renal stenting for renal artery coverage during endovascular aortic aneurysm repair. J Vasc Surg. 2009; 49:1100–1106.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download