Abstract
Spontaneous coronary artery dissection (SCAD) and woven coronary artery anomaly (WCAA) are relatively rare. A few of the previously reported woven coronary artery cases have involved in a single coronary artery. We present an unusual woven case involving all coronary arteries and two patient with SCAD. We have also reviewed the literature related to these disease, as they resemble one another.
Spontaneous coronary artery dissection (SCAD) and woven coronary artery anomaly (WCAA) are rare causes of acute coronary syndrome or sudden cardiac death. The incidence of SCAD is about 0.1% for patients who are referred for coronary angiography, and many believe this to be underestimated;1)2) on the other hand, the incidence of WCAA is unknown. WCAA is an extremely rare anomaly characterized by the branching of a major epicardial coronary artery into thin channels, which then merge again in a normal conduit.3) The clinical presentation of both diseases depend on the location and severity of coronary involvement. Herein, we present three cases and review of the literature.
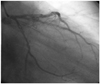
A 60-year-old female was presented to the cardiology department with stable angina pectoris that she had experienced for one year. She had been diagnosed with portal vein thrombosis due to protein C deficiency, hypertension and hyperlipidemia. She was on perindopril and warfarin. An electrocardiography (ECG) showed Q waves in the inferior leads. Cardiac catheterization showed a spiral dissection of the proximal right coronary artery (RCA), extending downwards to just below the right ventricle branch (Fig. 1), with distal Thrombolysis in Myocardial Infarction (TIMI)-3 antegrade flow. Ventriculography showed hypokinesis of the inferior wall, and the left ventricle ejection fraction (LVEF) was 45%. A 2.75×30 mm drug-eluting stent was successfully placed along the dissection, reducing the RCA to a single lumen (Fig. 2). Follow-up transthoracic echocardiography showed normal wall motions and ejection fraction. The patient was administered on warfarin, clopidogrel, perindopril and beta-blocker on discharge. At 12 weeks follow-up, she was well and without symptoms.
A 52-year-old man was presented with stable angina pectoris that lasted for two months. The patient was a diabetic and hypercholesterolemic smoker. He had experienced no cardiac events in the past. We performed coronary angiography because of the positive treadmill exercise test, and the coronary angiogram surprisingly revealed diffuse dissection that originated on the proximal bed of the second obtuse marginal-II branch and extending to its distal bed (Fig. 3). We did not perform coronary angioplasty due to technical difficulties and the narrowness of the ischemia-related artery. Hence, the patient was discharged on medical treatment with beta blocker and aspirin.
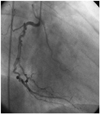
A 45-year-old male was presented to cardiology department with mild chest pain. The patient's history revealed a previous right carotid artery occlusion, hyperlipidemia, hypertension and severe smoking. The patient had been on clopidogrel, aspirin, and lisinopril for the last two months. The patient exhibited no ischemic ECG changes, but we determined an abnormal ST-segment depression on exercise testing. Subsequent coronary angiogram showed a dual twisted lumen in the proximal and medial segments of all coronary arteries (Figs. 4, 5, and 6), and ventriculography revealed normal wall motion and LVEF of 65%. We noticed that this patient had WCAA involving all coronary arteries. The patient's right carotid angiography showed an occlusion, and other vessels were normal on CT angiogram. There have been no cases reported in the literature with WCAA involving all coronary arteries. A workup for collagen disease was negative. The patient was evaluated for revascularization due to ischemic findings, but thallium-201 myocardial perfusion imaging did not show ischemia. We did not perform optical coherance tomography (OCT) due to the probability of coronary dissection or occlusion caused by guidewire or OCT catheter. The patient was discharged receiving 81 mg of aspirin and 75 mg of clopidogrel once a day. Smoking cessation was strongly encouraged.
Although it is a very rare clinical event, SCAD is most frequently associated with pregnant women or those in the postpartum and commonly seen in middle age, but is not infrequent in older patients.1)2)4) The precise aetiology and the mechanism of SCAD during pregnancy is uncertain. Peripartum episodes suggest hemodynamic stress of pregnancy, changes in connective tissue caused by high estrogen levels, and inflammatory changes due to infiltration of eosinophils in the adventitia of the vessel.4-6) A United States population-based study7) on all-cause acute myocardial infarction (AMI) in pregnancy estimated an incidence of one AMI in every 16129 pregnancies, with a mortality rate reported at 5.1%. Eighty percent of reported cases of SCAD occur in women with no history of heart disease or cardiovascular risk factors, and typically in the left anterior descending artery; men tend to present with RCA dissections. Of the women with SCAD, one-third are in the peripartum period.8-10) The disease also has higher prevalance among cases with collagen disorders, cocaine abuse, severe hypertension, smoking, and oral contraceptives.11)
Coronary artery dissection can be seen spontaneously as a consequence of coronary angiography, coronary intervention, cardiac surgery or chest trauma. The clinical presentation of SCAD relates to the extent and rate of dissection, as well as the degree of myocardial ischemia. Sudden cardiac death occurs in about 50% of cases, mostly in those with left main coronary artery dissection.12) For the diagnosis of SCAD, diagnostic coronary angiography is generally the standard method, as well as multidetector computerized coronary angiography. Intravascular ultrasound may also be useful, but experience with this technique is still very limited.
Spontaneous coronary artery dissection occurs by separation of layers of the arterial wall. When the separated intimal-medial layer pushes toward the true lumen of the vessel, the insuefficient flow of the coronary artery leads to distal myocardial ischemia and its clinical consequences. In some cases, the false lumen may also recover spontaneously and the true lumen may supply sufficient blood to the distal beds. Compression of the vessel lumen can result from haemorrhage and haematoma between the outer media and external elastic lamina.
According to the National Heart, Lung, and Blood Institute grade system developed by the Coronary Angioplasty Registry, dissections: can be classified A-F. Type A and B dissections are characterized by filling the defects on contrast injection where there is no or minimal persistence of contrast after the dye has cleared; type C dissections present as dye staining in an extraluminal cap; type D is seen as a spiral luminal defect; type E is detected as persistent luminal defects; and type F involves total luminal occlusion.13)
Treatment of SCAD has not been described well; management should be decided individually for each case, and includes medical therapy, percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery. At any rate, SCAD often exhibits with angina or a myocardial infarction necessitating urgent intervention. Some authors have advised that asymptomatic patients with a non-occlusive dissection could be managed conservatively.14) Fibrinolytics, which may provoke bleeding or dissection progression, should be avoided.15) Although, many dissections are not amenable to catheter interventions, PCI can be considered, especially for prominent major single-vessel disease with short segment involvement; this should be performed cautiously due to the possibility of false lumen or propagating the dissection distally. Medical management includes heparin, antiplatelets, and anti-ischaemic medications, such as beta-blockers and nitrates. Multivessel or left main SCAD should be managed with CABG.16)
Woven coronary artery anomaly is associated with multiple and twisted thin channels along the vessel in any coronary artery with a TIMI III blood flow distally; these then merge again in order to form the main lumen, after twisting along the coronary artery axis. The angiographic imaging of WCAA looks like an intracoronary thrombus or SCAD. The differential diagnosis between SCAD and WCAA may be very difficult for invasive cardiologists, especially in patients with single or two coronary artery involvement, as the occurence of WCAA is very rare. In the differential diagnosis, in case of the flow is suprisingly normal reflecting the extension of the apparent filling defects and/or there is not any ischemic event, WCAA should be taken into consideration. For better diagnosis, angiography should be performed using multiple projections with a careful radiological examination (digital zooming).
There has only been one case involving infarct-related WCAA in the literature,17) and no WCAA case in which PCI was performed. In a case with WCAA involving circumflex artery, reported by Kursaklioglu et al.18) no adverse coronary event occurred during the 5-year follow-up period. WCAA have been accepted as a benign entity and no adverse coronary event occured in the long-term follow-up period, due to TIMI III blood flow until now and it may demonstrate generally normal coronary reserve with a stress test because of normal blood flow distal to the anomalous segment.18-20) Some authors believed that WCAA may originate from SCAD and the twisting of thin channels probably causes intracoronary thrombus.20) As distinct from the cases reported in the literature,17-20) involving the right or circumflex artery alone, we present a very interesting WCAA, involving all coronary arteries. It would be helpful to have the examination by OCT, but we could not perform due to the risk of any procedural complication.
There are conflicting opinions in the literature about how WCAA occurs and whether it complicates any coronary ischemic event. It may originate from genetic disorders. More information is needed to increase the understanding of the nature of this malformation.
In conclusion, although woven coronary artery is an exceptionally rare coronary artery anomaly, all interventional cardiologists should keep SCAD and WCAA in mind during daily practice in the catheterization laboratory. Angiography with multiple projections should be obtained to make a differential diagnosis between iatrogenic dissection, thrombosis, or chronic total occlusion with bridging collaterals. In patients with SCAD and WCAA who present with ischemic symptoms, those unresponsive to medical management may benefit from surgical or percutaneous revascularization. We consider that extensive understanding of the woven coronary artery can reduce the risk of unnecessary intervention, which inherently exposes the patients. Although WCAA appear to be benign coronary anomalies without any major adverse cardiovascular events, we need more data to delineate the natural history of this malformation exactly.
Figures and Tables
Fig. 1
Right coronary angiogram demonstrating spontaneous dissection-left anterior oblique projection (case 1).
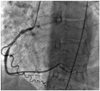
Fig. 2
Post percutaneous coronary intervention right coronary angiogram demonstrating successful stenting of the right coronary artery (case 1).
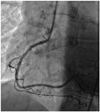
Fig. 3
Left coronary artery-right anterior projection. It shows a non-acute spontaneous dissection at the proximal segment and distal margins of the obtuse marginal-II branch. It was not appropriate for percutaneous coronary intervention (case 2).
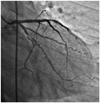
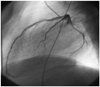
Fig. 4
Left coronary angiogram demonstrating woven coronary artery anomaly at the proximal segment of the left anterior descending artery and ramus intermedius artery and circumflex artery-left lateral projection (case 3).
References
1. Maeder M, Ammann P, Angehrn W, Rickli H. Idiopathic spontaneous coronary artery dissection: incidence, diagnosis and treatment. Int J Cardiol. 2005; 101:363–369.
2. Kamineni R, Sadhu A, Alpert JS. Spontaneous coronary artery dissection: report of two cases and a 50-year review of the literature. Cardiol Rev. 2002; 10:279–284.
3. Sane DC, Vidaillet HJ Jr. "Woven" right coronary artery: a previously undescribed congenital anomaly. Am J Cardiol. 1988; 61:1158.
4. Robinowitz M, Virmani R, McAllister HA JrU . Spontaneous coronary artery dissection and eosinophilic inflammation: a cause and effect relationship? Am J Med. 1982; 72:923–928.
5. Asuncion CM, Hyun J. Dissecting intramural hematoma of the coronary artery in pregnancy and the puerperium. Obstet Gynecol. 1972; 40:202–210.
6. Siegel RJ, Koponen M. Spontaneous coronary artery dissection causing sudden death. Mechanical arterial failure or primary vasculitis? Arch Pathol Lab Med. 1994; 118:196–198.
7. James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006; 113:1564–1571.
8. Thompson EA, Ferraris S, Gress T, Ferraris V. Gender differences and predictors of mortality in spontaneous coronary artery dissection: a review of reported cases. J Invasive Cardiol. 2005; 17:59–61.
9. DeMaio SJ Jr, Kinsella SH, Silverman ME. Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 1989; 64:471–474.
10. Dhawan R, Singh G, Fesniak H. Spontaneous coronary artery dissection: the clinical spectrum. Angiology. 2002; 53:89–93.
11. Hering D, Piper C, Hohmann C, Schultheiss HP, Horstkotte D. [Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection]. Z Kardiol. 1998; 87:961–970.
12. Cohen DE, Strimike CL. A case of multiple spontaneous coronary artery dissections. Catheter Cardiovasc Interv. 2000; 49:318–320.
13. Coronary artery angiographic changes after PTCA. Manual of operations. NHLBI PTCA Registry. 1985-6. p. 9.
14. Jorgensen MB, Aharonian V, Mansukhani P, Mahrer PR. Spontaneous coronary dissection: a cluster of cases with this rare finding. Am Heart J. 1994; 127:1382–1387.
15. Koga T, Sakamoto A, Nakamura Y, et al. Circumferential spontaneous coronary artery dissection in an elderly man: a case report. Angiology. 1998; 49:83–86.
16. Vrints CJ. Spontaneous coronary artery dissection. Heart. 2010; 96:801–808.
17. Soylu K, Meric M, Zengin H, Yüksel S, Kaya MG. Woven right coronary artery. J Card Surg. 2012; 27:345–346.
18. Kursaklioglu H, Iyisoy A, Celik T. Woven coronary artery: a case report and review of literature. Int J Cardiol. 2006; 113:121–123.
19. Iyisoy A, Celik T, Yuksel UC, Isik E. Woven right coronary artery: a case report and review of the literature. Clin Cardiol. 2010; 33:E43–E45.
20. Martuscelli E, Romeo F, Giovannini M, Nigri A. Woven coronary artery: differential diagnosis with diffuse intracoronary thrombosis. Ital Heart J. 2000; 1:306–307.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download