Introduction
Coronary artery fistulas (CAF) are rare cardiac anomalies, the angiographic incidence of which is 0.17-0.25%.1) The majority of CAF originate from the right coronary artery (RCA), but they less commonly may originate from the left coronary or from multiple arteries.2) Multiple CAF account for about 10% to 16% of all cases, whereas those originating from both coronary arteries are extremely rare and their impact on patient's clinical outcomes and hemodynamics are less known.3) The clinical symptoms and signs related to multiple CAFs are variable. Most patient are asymptomatic but they may sometimes present with congestive heart failure in infants and chest pain due to coronary steal syndrome in adults.4) It is not well known whether there are clear associations between significant endothelial dysfunction and subsequent significant coronary artery spasm (CAS) and CAF as a coronary anomaly.5) Spontaneous regression of CAF has been reported in few cases, almost all of which were infants.6)7)
We present a case of 41-year-old woman who presented with ischemic chest pain at rest, and who was initially diagnosed with multiple CAF with severe CAS by coronary angiography and acetylcholine (Ach) provocation test. Following intensive antianginal medical therapy, she showed spontaneous regression of one of the fistulas at two-year follow-up coronary angiography.
Case
A 41-year-old woman presented with a several week history of resting ischemic chest pain. She had no specific past medical history. On physical examination, there was no pathologic murmur sound on auscultation, and the remaining physical examination findings were not remarkable. On further questioning, she reported experiencing episodes of resting ischemic chest pain over the previous few years. The routine laboratory findings were not remarkable. Chest X-ray showed no signs of pulmonary edema and the cardiothoracic ratio was 42%. Electrocardiogram showed normal sinus rhythm without ST segment change. Initial transthoracic echocardiography showed mild tricuspid regurgitation with neither wall motion nor left ventricular systolic function abnormality. A treadmill stress test was done during which the patient exercised for ten minutes following the Bruce protocol and maximal workload was attained (12 METs) without chest pain. However, electrocardiogram at peak exercise level showed ST depression of about 2 mm in leads II, III and aVF.
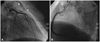
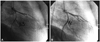
Based on the patient's symptoms and the results of the treadmill test, we decided to perform coronary angiography. Baseline coronary angiography revealed multiple small fistulas which originated from the proximal left main (LM) coronary artery to the pulmonary artery (PA), from the proximal left anterior descending artery (LAD) to PA and from the proximal RCA to PA (Fig. 1). Because the coronary angiography showed no significant lesions in the major epicardial coronary arteries, we performed an incremental intracoronary Ach provocation test with A1 (20 ug), A2 (50 ug) and A3 (100 ug) into the left coronary artery. This showed significant diffuse vasoconstriction in the LAD and left circumflex artery with ischemic chest pain at dose A3 (Fig. 2).
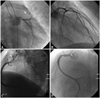
In light of the multiple coronary AVF, we directly performed right heart catheterization for functional assessment. There was minimal oxygen step-up between the inferior vena cava level (80.5%) and those of the pulmonary arteries (70.1%) and the right atrium (70.4%). The calculated Qp/Qs was 0.98. For further investigation, computed tomography angiography was carried out for three dimensional understanding, which showed similar results as compared to the coronary angiography findings (Fig. 3).
Based on the results of coronary angiography and right heart catheterization, we decided to manage conservatively for the multiple CAFs combined with significant CAS, treating with antianginal medication including nicorandil (Sigmart®, JW Pharmaceutical, Korea) and isosorbide dinitrate (Isoket®, KPP, Korea) instead of selective embolization of the major coronary AVF. After this intensive antianginal medication treatment, the patient's ischemic symptoms improved and maintained a stable condition.
Two years later, the patient visited the emergency room with ischemic chest pain at rest and follow-up coronary angiography was performed for definite evaluation. Coronary angiography showed no significant interval change as compared with the previous results especially in the AVF from LM to PA and that from LAD to PA. However, the main AVF from the proximal RCA to PA had spontaneously regressed (Fig. 4). After careful history taking, we ascertained that the patient had not taken antianginal medicine several days prior to visiting the emergency room. We accordingly restarted antianginal treatment, and her symptoms were improved and stabilized.
Discussion
Coronary artery fistula is very rare anomaly, the natural history of which remains to be understood. Most CAF originate from the RCA, and drain to the right heart (right atrium, right ventricle, PA). The majority of anomalies involve one coronary artery. Cases involving more than two arteries are extremely rare.3) Approximately 5% of total CAF cases originate from bilateral coronary arteries.8) Our report concerns a representative bilateral CAF case in which fistulas drained into the PA.
Coronary artery fistula may induce microcirculation and chronic volume overload and give rise to reactive myocardial hypertrophy, which may present as typical or atypical chest pain.9) Furthermore, shunt flow from the coronary artery to relatively less resistant fistula, which is called coronary steal syndrome, may induce symptoms.10) In our case, the patient's chest pain might have partly originated from the above mechanisms, but more likely originated from combined significant CAS. We could indirectly clinically confirm that significant CAS may have been the main reason for this patient's symptoms because of the chest pain characteristics (resting pain typically shown in patients with vasospastic angina) and the excellent response to antianginal medication.
There is a report regarding mutation in the endothelial nitric oxide synthetase gene, which was found to be significantly increased in vasospastic angina patients.11) Further, Leu12) found that patients with coronary arteriovenous malformation had histological change in coronary vessels, which showed disappearance in the elastic layer and inner smooth muscle layer and change in the endothelial layer. If we put together these findings, we can suggest that decreased nitric oxide production due to endothelial dysfunction may be the key role of vasospasm in CAF. We are now conducting prospective registry to observe whether there is a clear association between CAF and CAS in a series of patients, and we can see some close trends between these two disease subsets. However, more extensive follow-up data with a larger study population over a longer period will be needed to identify the precise mechanisms involved and to make a final conclusion.
Management of CAF is controversial, especially in asymptomatic patients with non-significant shunt flow. Yet CAF closure should be considered in asymptomatic patients with elevated shunt ratios to prevent the development of symptoms or complications.1) Direct ligation has previously been the therapeutic choice for closure of CAF, however, transcatheter closure has been widely used in recent years and is recognized as a safe procedure. Furthermore, patients receiving conservative therapy should be monitored closely for development of symptoms and possible complications.
Spontaneous closure of CAF is extremely rare, and there are only a few reported cases in adults.13-15) In our case, AVF that originated from the proximal RCA to PA had regressed over a period of two years. The mechanism of partial regression is unclear, but thrombosis or sclerotic change in CAF may be considered possible causes. The effect of shunt flow may be a possible mechanism, in terms of shear stress-induced intimal damage in the narrow fistulous communication.13) We speculate that improved major epicardial and microvascular flow due to antianginal medications might be another causative factor inducing thrombotic occlusion in AVF by decreasing flow to the abnormal shunt which has preexisting intimal damage.
In conclusion, our patient showed multiple small CAF without significant coronary artery disease but combined significant CAS was confirmed by intracoronary Ach provocation test, which was then successfully and safely managed by medical therapy. One of the major AVF had regressed spontaneously without mechanical intervention at two year follow-up. This particular case suggests that significant CAS can be an important cause of ischemic chest pain in patients with CAF, especially in Asian populations. Therefore, routine mechanical management by elective CAF ligation or embolization should be avoided and carefully considered in selective CAF patients, especially those with combined CAS.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download