Abstract
Stent thrombosis is a very serious problem after drug-eluting stent (DES) implantation even though its incidence is about or less than 1%. As the clopidogrel resistance is expected to play an important role in the occurrence of stent thrombosis, new anti-platelet agents overcoming this issue can give us another choice. We experienced a case of a 58-year-old male with successful prasugrel rescue therapy in a patient with clopidogrel resistance who had recurrent stent thrombosis following DES implantation.
Stent thrombosis is a rare but fatal complication of drug eluting stent (DES). Although dual antiplatelet therapy with aspirin and clopidogrel significantly reduced the occurrence of stent thrombosis, late stent thrombosis still occurs; many other factors could be attributable to the occurrence.1)2) Of these, clopidogrel resistance is regarded as one of the main reasons for the occurrence of DES thrombosis. Therefore, new antiplatelet agents have been developed recently and are reported to have better outcomes.3) We report a case of recurrent DES thrombosis with clopidogrel resistance successfully rescued by prasugrel.
A 58-year-old non-smoker male who had no specific medical history visited the emergency room with typical angina on April 2006. Coronary angiographic findings showed significant narrowing at 3 major epicardial arteries (Fig. 1A and B). Percutaneous coronary intervention with 3.0×23 mm-sized sirolimus-eluting stent (CYPHER®; Cordis Corp., Miami Lakes, FL, USA) at the mid portion of the left anterior descending artery (m-LAD) and 2.75×18 mm-sized sirolimus-eluting stent at the distal portion of left circumflex artery (d-LCx) were performed (Fig. 1C). He received triple antiplatelet drugs (aspirin 100 mg daily, clopidogrel 75 mg daily, cilostazol 100 mg twice daily). After that, cilostazol and clopidogrel were discontinued on July 2006 (3-month use) and on June 2009 (37-month use), respectively. Three weeks after clopidogrel discontinuation, he felt typical angina and was diagnosed with non-ST-segment-elevated myocardial infarction (creatine kinase-MB 5.64 ng/mL, Troponin-T 0.019 ng/mL). On the coronary angiography, intraluminal thrombi in the distal portion of m-LAD stent and totally occluded d-LCx stent were noted (Fig. 1D, E and F). Percutaneous coronary intervention with 3.0×20 mm-sized balloon was performed on m-LAD and d-LCx stents as well as on the distal portion of LAD. Thereafter, triple antiplatelet therapy, including cilostazol 100 mg twice daily, was restarted. The patient had no further chest pain and cilostazol and clopidogrel were discontinued on March 2010 (9-month use) and on June 2010 (12-month use), respectively.
However, he visited the emergency room again on Jan 2011 because of a severe ongoing chest pain. Electrocardiogram showed ST elevation (>1 mm) on the anterior (V 1-3) lead. Emergency coronary angiography was performed and both m-LAD and d-LCx stents were totally obstructed on the angiogram (Fig. 2A and B). Thrombus aspiration (Thrombuster II; Kaneka Medix Corporation, Osaka, Japan) and balloon dilation with 3.0×15 mm-sized balloon were performed on both m-LAD and d-LCx stents (Fig. 2C). Because of the drop of blood pressure during the procedure (80/50 mm Hg), intraaortic balloon pump (AutoCAT2 WAVE-Fiber Optic; Arrow International Inc., Reading, PA, USA) and percutaneous cardiopulmonary support (CAPIOX EBS; Terumo Corporation, Tokyo, Japan) were applied. Transthoracic echocardiogram was performed right after the procedures showed a reduced left ventricular ejection fraction of 25% with anterior and anteroseptal wall akinesis. Triple antiplatelet therapy was conducted again. After that, the patient was stable and all the other devices were removed; he was sent to the general ward. However, thirteen days post procedure, he experienced a severe resting pain again. The resting electrocardiogram showed a ST segment elevation on V 1-6 leads. On the emergency angiogram, previously treated LAD and LCx stents were occluded again (Fig. 2D and E).
Percutaneous coronary intervention with 3.0×13 mm-sized balloon was performed on m-LAD and d-LCx stents (Fig. 2F). Due to suboptimal revascularization flows, 3.0×30 mm-sized zotarolimus-eluting stent implantation (Endeavor® Sprint; Medtronic Cardio-Vascular, Minneapolis, MS, USA) was performed on m-LAD. The day before the last event, the platelet function test with Verify-Now-P2Y12® rapid analyzer (Accumetrics Inc., San Diego, CA, USA) showed inadequate platelet inhibition results {280 P2Y12-receptor Reaction Units (PRU), 14% inhibition}.
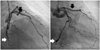
Finally, clopidogrel was switched with prasugrel 10 mg daily followed by a 60 mg loading. Six days later, the platelet function test showed an adequate platelet inhibition (9 PRU, 98% inhibition). After discharge, cilostazol was discontinued on May 2011 (4-month use) and the platelet function test still showed a good response to the dual antiplatelet with aspirin and prasugrel (92 PRU, 70% inhibition). After a nine-month follow-up, the coronary angiography showed a patent both in LAD and LCx stents. Currently, dual antiplatelet drugs with aspirin and prasugrel were maintained without any chest pain (13-month use) (Fig. 3).
Although dual antiplatelet therapy with aspirin and clopidogrel is the standard medication in patients with DES implantation, the unmet needs still exist and fatal complication occurs. Particularly, clopidogrel resistance, regarded as one of the major factors for stent thrombosis, has been reported to occur in 30-40% of patients.4-7) To overcome clopidogrel resistance, a dose-up of clopidogrel or triple antiplatelet therapy with cilostazol has been recommended. However, recent clinical trials, such as GRAVITAS (Gauging Responsiveness with A VerifyNow assay-Impact on Thrombosis And Safety) did not show a better prognosis than the existing standard-dose dual antiplatelet.8) Although some clinical trial described that triple antiplatelet therapy including cilostazol has a beneficial effect on ischemic complications, there are still many debates.9)10) Under this situation, new anti-platelet agents give us a new choice. Of these, prasugrel, which is hydrolysed by esterases into an intermediate precursor and whose activation is not involved with oxidation by the enzyme CYP2C19,11) can consistently and rapidly inhibit ADP-induced platelet aggregation than clopidogrel.12) Prasugrel was significantly superior to clopidogrel for the reduction of ischemic events, including stent thrombosis in TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis.3) Also, some studies reported that prasugrel achieved greater inhibition of platelet aggregation in clopidogrel resistance patients.13)
Our case revealed the recurrent stent thrombosis events despite dual or triple antiplatelet therapy including cilostazol. Many factors might be attributable to the occurrence of stent thrombosis. In particular, new stent implantation (Endeavor® Sprint) at m-LAD could make a beneficial contribution for stent thrombosis through the hemodynamic improvement in the present case. However, d-LCx stent (CYPHER®) with balloon-only angioplasty has also shown a good patency in a follow-up angiography. Thus, rescued clopidogrel resistance could be the main reason rather than the hemodynamic improvement. After the change of antiplatelet into prasugrel, clopidogrel resistance was successfully rescued and no thrombotic event has been observed until now. In conclusion, for the prevention of recurrent DES thrombosis, dual antiplatelet with aspirin and prasugrel could be one of the potential options in patients with clopidogrel resistance.
Figures and Tables
Fig. 1
At the first admission, left coronary artery angiography showed tubular eccentric 80% luminal narrowing of the mid portion of the left anterior descending artery (m-LAD) (black arrow) and tubular eccentric 90% luminal narrowing of the distal portion of left circumflex artery (d-LCx) (white arrow) (A and B). Successful percutaneous coronary intervention with stent insertion was performed on m-LAD and d-LCx (C). At the second admission, thrombi was observed in distal portion of m-LAD stent (black arrow). Also, totally occluded d-LAD lesion (narrow white arrow) and d-LCx stent (white arrow) were noted (D, E and F).
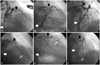
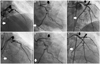
Fig. 2
At the third admission, both mid portion of the left anterior descending artery (m-LAD) (black arrow) and distal portion of left circumflex artery (d-LCx) (white arrow) stents were totally obstructed with thrombi (A and B). After the procedure, revascularization flow was recovered at both stent areas (C). In re-stent thrombosis event at the third admission, there were no flows at both stent areas in the emergency angiography (D and E). After balloon dilation on m-LAD and d-LCx stents, suboptimal revascularization flows were observed at both stents (F).
References
1. Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007; 369:667–678.
2. Honda Y, Fitzgerald PJ. Stent thrombosis: an issue revisited in a changing world. Circulation. 2003; 108:2–5.
3. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. 2008; 51:2028–2033.
4. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Identification of low responders to a 300-mg clopidogrel loading dose in patients undergoing coronary stenting. Thromb Res. 2005; 115:101–108.
5. Gurbel PA, Tantry US. Drug insight: Clopidogrel nonresponsiveness. Nat Clin Pract Cardiovasc Med. 2006; 3:387–395.
6. Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005; 45:246–251.
7. Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2011; 57:e215–e367.
8. Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011; 305:1097–1105.
9. Douglas JS Jr, Holmes DR Jr, Kereiakes DJ, et al. Coronary stent restenosis in patients treated with cilostazol. Circulation. 2005; 112:2826–2832.
10. Lee SW, Park SW, Hong MK, et al. Triple versus dual antiplatelet therapy after coronary stenting: impact on stent thrombosis. J Am Coll Cardiol. 2005; 46:1833–1837.
11. Rehmel JL, Eckstein JA, Farid NA, et al. Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug Metab Dispos. 2006; 34:600–607.
12. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357:2001–2015.
13. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006; 27:1166–1173.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download