Abstract
We describe a 64-year-old male patient with panhypopituitarism who experienced polymorphic ventricular tachycardia (VT) associated with long QT intervals. The panhypopituitarism developed as a sequelae of radiation therapy administered 20 years prior to his current presentation and was recently aggravated by urinary tract infection with sepsis. In this case, polymorphic VT was resistant to conventional therapy (including magnesium infusion), and QT prolongation and T wave inversion were normalized after the administration of steroid and thyroid hormones. Thyroid hormone is generally known to be associated with torsades de pointes (TdP), but steroid or other hormones may also provoke TdP. Hormonal disorders should be considered as a cause of polymorphic VT with long QT intervals. Some arrhythmias can be life-threatening, and they can be prevented with supplementation of the insufficient hormone.
Torsades de pointes (TdP) may occur as a consequence of acquired QT interval prolongation, resulting from the use of various medications, liquid protein diets, intracranial events, bradyarrhythmias, electrolyte disturbances, and hormonal disorders.1-4) Because many cases of polymorphic ventricular tachycardia (VT) have been reported, and the condition is resistant to antiarrhythmic drug therapy, it is important to determine the primary cause of long QT intervals and the way to correct it.
We experienced a case of TdP associated with panhypopituitarism, which developed as a sequelae of radiation therapy administered 20 years prior to the current presentation and was recently aggravated by urinary tract infection (UTI) sepsis. We hypothesized that TdP may have been caused by panhypopituitarism.
A 64-year-old man was admitted to our hospital from shock caused by a UTI. The patient had a medical history of local radiation therapy on the surgical area after undergoing functional endoscopic sinus surgery for a nasal tumor (left maxillary cancer, squamous cell carcinoma) 20 years ago. The radiation therapy included the pituitary gland. He was followed up for 5 years after the surgery, but the follow-up was discontinued, as there was no possibility of recurrence. He had lived without any discomfort. He took tamsulosin for benign prostatic hypertrophy and used Spiriva® (Spiriva®, Boehringer Ingelheim GmbH, Germany) inhaler for chronic obstructive pulmonary disease.
At admission, the patient's blood pressure was 70/40 mm Hg, his pulse rate was 88 beats/min, his respiration rate was 26 times per minute, and his body temperature was 38.0℃. The patient was managed for septic shock with antibiotics, including ceftazidime, in the intensive care unit (ICU), and his blood pressure stabilized. On the 4th day of ICU admission, non-sustained polymorphic VT (TdP) occurred for 20 seconds. The VT disappeared spontaneously (Fig. 1). The patient indicated that he briefly felt drowsy, but his blood pressure after the event was 110/70 mm Hg. Laboratory tests reported the following findings: glucose 141 mg/dL (70-110 mg/dL); Na+ 144 mEq/L (135-145 mEq/L); K+ 3.8 mEq/L (3.5-5.5 mEq/L); corrected Ca2+ 8.6 mg/dL (8.4-10.2 mg/dL); and Mg2+ 1.4 mg/dL (1.9-2.5 mg/dL).
The patient was treated with a loading dose of Mg (2 g) and a maintenance dose of Mg (1 g/day for 8 days). After the administration of magnesium, the patient's laboratory parameters were as follows: Na+ 144 mEq/L (135-145 mEq/L); K+ 4.2 mEq/L (3.5-5.5 mEq/L); corrected Ca2+ 8.8 mg/dL (8.4-10.2 mg/dL); and Mg2+ 2.4 mg/dL (1.9-2.5 mg/dL).
Although his Mg level normalized, the QTc interval prolongation and T wave inversion persisted, and TdP recurred several times. When TdP appeared, the patient's blood pressure was stable, but he became stuporous. Therefore, echocardiography, coronary angiography, and temporary ventricular overriding pacing with a ventricular rate of 90 beats per minute were performed. Echocardiography demonstrated a left ventricular ejection fraction of 54% with no regional wall motion abnormality, and no abnormal findings were observed during coronary angiography. A thyroid function test showed reduced values: T3 35 ng/dL (65-150 ng/dL); free T4 0.36 ng/dL (0.78-1.54 ng/dL); and thyroid-stimulating hormone (TSH) 0.27 µIU/mL (0.35-5.5 µIU/mL).
Luteinizing Hormone (LH) and follicle stimulating hormone (FSH) were measured to evaluate abnormal thyroid functioning. The TSH releasing hormone stress test demonstrated no increase in TSH (0.22 µIU/mL; range 0.35-5.5 µIU/mL), LH (0.9 mIU/mL; range 1.0-5.3 mIU/mL); and FSH (72.5 mIU/mL; range 1.1-13.5 mIU/mL). After panhypopituitarism was diagnosed, brain CT and brain MRI with enhancement were performed, and a small pituitary gland was found. Before treatment, the average urine output was 4 L daily. The patient's serum and urine osmolarity were 300 mOsm/kg (275-295 mOsm/kg) and 247 mOsm/kg (300-900 mOsm/kg), respectively.
To correct the pituitary gland dysfunction, a steroid (prednisolone, 7.5 mg), thyroid hormone (levothyroxine, 100 µg), and desmopressin (Minirin nasal spray®, 5 µg bid) were administered. After one week of hormone treatment, the QTc prolongation and T wave inversion decreased mildly. After 4 weeks of steroid hormone, thyroid hormone, and desmopressin treatment, electrocardiography showed a normal QTc range (430 ms), and the T wave inversion disappeared (Table 1).
TdP did not recur. After desmopressin administration, the patient's symptom of diabetes insipidus improved, and his urine output decreased to 1-2 L daily. His serum osmolarity normalized to 288 mOsm/kg (275-295 mOsm/kg), and his urine osmolarity increased to 544 mOsm/kg (300-900 mOsm/kg).
We described a case of panhypopituitarism associated with QT prolongation and polymorphic VT.
The hormone most widely known to be associated with TdP is thyroid hormone. The physiological chronotropic response and normal tension of the heart muscle in the diastolic phase depend on the proper expression of tri-iodothyronine in the heart cells and its stimulating influence on Na+-K+ ATPase and Ca2+ ATPase in the endoplasmic reticulum. Normal heart contractility is also related to proper tri-iodothyronine-stimulated transcription of the myosin heavy-chain alpha gene and inhibition of the heavy-chain beta gene. Moreover, proper tri-iodothyronine expression in the cardiac muscle affects the number of β-adrenergic receptors and their sensitivity to catecholamines. Profound hypothyroidism and decreased expression of tri-iodothyronine in the heart cells may cause a worsening of cardiac contractility, a decreasing heart rate and a slowing of the conduction of electrical stimuli in the heart muscle. This may be the reason for bradycardia and prolongation of the QT interval. As a result, life threatening arrhythmias may occur, such as torsade des pointes-type tachycardia.5)6)
In addition to thyroid hormone, steroid hormones are also known to be associated with TdP.7-9) Although the pathogenesis remains unknown, hypopituitarism has been reported to be commonly associated with electrocardiac abnormalities. T wave inversion was the most common finding, and prolongation of the QT interval was the second most common finding.10) With ECG changes, there were some reports of polymorphic VT with hypopituitarism. Although the mechanism remains unclear, with glucocorticoid deficiency, an intracellular and extracellular electrolyte imbalance in myocytes and histopathological changes in the myocardium are thought to play a role in this disorder. It was reported that glucocorticoids up-regulate Kv 1.5 K+ channel gene expression in the rat ventricle.11) Additionally, Iga et al.12) suggested that catecholamine release induced by hypoglycemia might cause arrhythmia or abnormal wall motion of the left ventricle in patients with adrenal insufficiency.
Because it is known that the female gender is associated with a higher risk for polymorphic VT and sudden cardiac death in adult patients with congenital long QT syndrome (LQTS)-type 1 and type 2, there is a potential role of the sex hormones in modulating the arrhythmogenic risk in LQTS.13) A previous animal study indicated that the occurrence of VT and sudden cardiac death was abolished with oral progestins in transgenic LQT2 rabbit model.14) However, there was no clear relationship between TdP and sex hormone and acquired LQTS.
In our case, we were unable to determine which hormonal effect was the most important, because thyroid hormone and glucocorticoid hormone were administered together.
To the best of our knowledge, this is the only report of an association between TdP and panhypopituitarism that developed as a sequelae of radiation therapy. Previous reports described cases of TdP with hypopituitarism, caused by Sheehan syndrome and the sequelae of hemorrhagic fever with renal syndrome.8)9) With the administration of steroid hormone and thyroid hormone, the patient's QTc prolongation and inverted T wave showed improvement, and TdP did not recur.
Hormonal disorders should be considered as a cause of polymorphic VT with long QT intervals. With supplementation of the deficient hormones, this life-threatening arrhythmia can be cured.
Figures and Tables
Fig. 1
Electrocardiography conducted at the hospital on day 4 showed a section of TdP rhythm during a 20-second period.
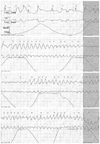
Table 1
Thyroid function test and electrocardiography conducted after the administration of steroid (prednisolone; 7.5 mg), thyroid hormone (synthyroxine; 100 ug daily replacement), and desmopressin (Minirin nasal spray ®; 5 µg bid). After hormone replacement treatment, the QTc prolongation disappeared

References
1. Roden DM. A practical approach to torsade de pointes. Clin Cardiol. 1997; 20:285–290.
2. Molokhia M, Pathak A, Lapeyre-Mestre M, et al. Case ascertainment and estimated incidence of drug-induced long-QT syndrome: study in Southwest France. Br J Clin Pharmacol. 2008; 66:386–395.
3. Sanaei-Zadeh H, Shahmohammadi F, Zamani N, Mostafazadeh B. Can death unrelated to secondary causes be predicted in intubated comatose tricyclic antidepressant-poisoned patients? Clin Toxicol (Phila). 2011; 49:379–384.
4. Hume-Smith HV, Sanatani S, Lim J, Chau A, Whyte SD. The effect of propofol concentration on dispersion of myocardial repolarization in children. Anesth Analg. 2008; 107:806–810.
5. Shojaie M, Eshraghian A. Primary hypothyroidism presenting with Torsades de pointes type tachycardia: a case report. Cases J. 2008; 1:298.
6. Schenck JB, Rizvi AA, Lin T. Severe primary hypothyroidism manifesting with torsades de pointes. Am J Med Sci. 2006; 331:154–156.
7. Nishizawa S, Nakamura T, Hamaoka T, Matsumuro A, Sawada T, Matsubara H. Lethal arrhythmia and corticosteroid insufficiency. Am J Emerg Med. 2009; 27:1167.e1–1167.e3.
8. Kim NH, Cho JG, Ahn YK, et al. A case of torsade de pointes associated with hypopituitarism due to hemorrhagic fever with renal syndrome. J Korean Med Sci. 2001; 16:355–359.
9. Izumi C, Inoko M, Kitaguchi S, et al. Polymorphic ventricular tachycardia in a patient with adrenal insufficiency and hypothyroidism. Jpn Circ J. 1998; 62:543–545.
10. Hartog M, Joplin GF. Effects of cortisol deficiency on the electrocardiogram. Br Med J. 1968; 2:275–277.
11. Takimoto K, Levitan ES. Glucocorticoid induction of Kv1.5 K+ channel gene expression in ventricle of rat heart. Circ Res. 1994; 75:1006–1013.
12. Iga K, Hori K, Gen H. Deep negative T waves associated with reversible left ventricular dysfunction in acute adrenal crisis. Heart Vessels. 1992; 7:107–111.
13. Sauer AJ, Moss AJ, McNitt S, et al. Long QT syndrome in adults. J Am Coll Cardiol. 2007; 49:329–337.
14. Odening KE, Choi BR, Koren G. Sex hormones and cardiac arrest in long QT syndrome: does progesterone represent a potential new antiarrhythmic therapy? Heart Rhythm. 2012; 9:1150–1152.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download