Abstract
Background and Objectives
Intravascular ultrasound (IVUS) is helpful during percutaneous coronary intervention (PCI), because it can be used to confirm good apposition or optimal expansion of stents. In this study, we compared angiographic result as well as clinical outcomes between two different strategies of IVUS-guidance, the selective vs. the routine.
Subjects and Methods
The study population consisted of 279 patients undergoing electric and emergency intracoronary implatation of TAXUS stent from August 2003 through September 2006. For this study, we divided physicians into two groups; doctors to perform PCI under 'routine' IVUS-guidance vs. PCI under 'selective' IVUS-guidance. Among a total of 279 patients (384 lesions) who underwent PCI with TAXUS stent, 87 patients underwent the procedure under the strategy of 'routine' IVUS-guidance, whereas 192 patients under 'selective' IVUS-guidance.
Results
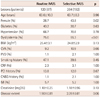
The baseline clinical features of the patients are similar between the two groups. The actual rate of IVUS usage was 89.2% in the routine group and 68.2% in the selective group (p<0.01). A high rate of adjunctive ballooning was determined as a remarkable procedure-related parameter which was comparable between the two groups (72.5% vs. 76.1% in routine vs. selective, p=0.57). The minimal lumen diameter at immediate post-PCI was significantly larger in the routine IVUS group than that in the selective group (2.58 mm vs. 2.48 mm, p=0.03). However, the difference disappeared during the follow-up period (1.98 mm vs. 1.98 mm, p=0.94). Clinical outcomes at 1 year were not different between the two groups.
Intravascular ultrasound (IVUS) has been known to be a useful tool during coronary angiography (CAG) and percutaneous coronary intervention (PCI).1-6) It helps us to see directly the coronary vessel wall including atheroma, while angiography shows us only luminography. Moreover, after stent deployment, IVUS can be used to confirm whether there is good apposition between the vessel wall and the stent. Suboptimal deployment may give rise to subacute thrombosis or restenosis in both bare-metal stents (BMS) and drug-eluting stents (DES).7-11) Based on the information obtained by IVUS, we could repeat inflation with a balloon at higher pressure, resulting in improved stent apposition and a wider luminal cross section area.
Previous studies have compared clinical outcomes of PCI with and without IVUS-guidance.12-16) In the real world practice, however, coronary interventionists usually use IVUS 'selectively' only when it is judged to be needed. There has not been a study comparing clinical and angiographic outcomes between the two different strategies: 'routine' IVUS-guidance vs. 'selective' IVUS-guidance in the DES era. The purpose of this study was to determine whether the selective use of IVUS is comparable to the routine use in terms of angiographic as well as clinical outcomes.
This was a single-center retrospective analysis of native coronary vessel DES implantations from August 2003 through September 2006 in the DES registry of Seoul National University Hospital. The study population consisted of patients with coronary artery disease who underwent elective and emergency coronary artery stenting of the native coronary vessel with a paclitaxel-eluting stent to exclude the bias coming from the use of different stents. The study population was divided into 2 groups: routinely IVUS-guided vs. selectively IVUS-guided. The routinely IVUS-guided group consisted of patients in whom stent implantation was performed with IVUS guidance as far as it was possible to perform IVUS examination even if angiographic findings showed good appearance. In contrast, the selectively IVUS-guided group was comprised of patients in whom IVUS was used after being judged to be needed by the operators. Judgements were made when the operators were not confident whether malapposition, edge tear or underexpansion was present or not. Patients were followed up for 1-year for mortality, myocardial infarction (MI), stent thrombosis, target lesion revascularization (TLR) and target vessel revascularization (TVR). For patients who underwent more than one procedure, the first intervention was used for the analysis. The local institutional review board approved this study.
Clinical data at baseline and follow-up were obtained from outpatient medical records or telephone interviews. The major adverse cardiac event (MACE) of this study was the composite of death, MI, stent thrombosis and TVR including TLR. MI was defined as new significant electrocardiographic Q waves or a creatinine kinase-MB isoenzyme level >3 times the upper limit of normal.
Coronary angiograms taken at baseline, at completion of stenting, and at follow-up were analyzed in our angiographic core laboratory (Seoul National University Hospital, Seoul, Korea).
The timing of IVUS imaging was decided by the operator (i.e., preimplantation or postimplantation or both). The vessel including the stented area was imaged with a mechanically rotated IVUS transducer. The imaging catheter was withdrawn with an automatic pull-back device at 0.5 mm/s. The operator concluded optimal stent deployment when there were full apposition, adequate acute gain and the absence of edge tears. If underexpansion, malapposition or edge tear was noted on IVUS images, further balloon inflations were performed with the use of either higher pressure, larger balloon, or an additional stent. After further intervention, IVUS imaging was repeated to confirm optimal stent deployment.
All data are expressed as mean value±SD or as number (percentages). The baseline characteristics of the groups and follow-up data were compared by the t-test for continuous variables and by the χ2 statistic for noncontinuous variables. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 12.0 (SPSS Inc., Chicago, IL, USA), and a p<0.05 was considered statistically significant.
The study population consisted of 279 patients undergoing elective and emergency intracoronary implantation of TAXUS stent. Of these patients, 87 patients (120 lesions) underwent PCI under routine IVUS-guidance, whereas 192 patients (264 lesions) under selective IVUS-guidance. The two groups were well balanced in terms of baseline clinical characteristics, except that female patients were more common and dyslipidemia was less common in the selectively IVUS-guided group, which is presented in Table 1. The angiographic and procedural data are shown in Table 2. The proportion of the left anterior descending artery was higher in the selectively IVUS-guided group, and the proportion of the left circumflex artery was higher in the routinely IVUS-guided group. Implanted stent number per lesion was well balanced between both groups. The number of stents per lesion was 1.2 in the routinely IVUS-guided group and 1.15 in the selectively IVUS-guided group (p=0.33). However, stent length was longer in the routinely IVUS-guided group. Interestingly, both groups underwent post-balloon dilatation at comparable frequencies (72.5% vs. 76.1%, p=0.57). Even in patients under the routine strategy, the rate of IVUS imaging was not 100% but about 90%, which is still significantly higher than the rate in those under the selective strategy (89.2% vs. 68.2%, p<0.01).
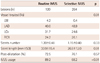
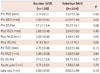
There was no difference in baseline reference vessel diameter and percent diameter stenosis (2.71 mm vs. 2.67 mm, p=0.50; 67.2% vs. 70.2%, p=0.06, respectively) (Table 3). However, minimal lumen diameter was slightly wider in the routinely IVUS-guided group (0.88 mm vs. 0.79 mm, p=0.05). In the case of the selectively IVUS-guided group, there were no differences in baseline reference vessel diameter and percent diameter stenosis between lesions with IVUS and lesions without IVUS (2.69 mm vs. 2.61 mm, p=0.28; 69.1% vs. 72.1%, p=0.12, respectively). After stent implantation, minimal lumen diameter was wider in the routinely IVUS-guided group (2.58 mm vs. 2.48 mm, p=0.03). In addition, percent diameter stenosis was smaller in the routinely IVUS-guided group (9.8% vs. 12.1%, p=0.05). Nonetheless, reference vessel diameter and acute gain did not show any difference (2.88 mm vs. 2.84 mm, p=0.45; 1.71 mm vs. 1.69 mm, p=0.75, respectively). Of the 384 lesions which received stent implantation, follow-up angiography was performed in 345 (89.8%). Of these, 336 (87.5%) angiograms were determined to be technically sufficient for analysis. At 6 to 9 angiographic follow-up, there were no statistically significant differences between the routinely IVUS-guided and selectively IVUS-guided groups with respect to reference vessel diameter (2.70 mm vs. 2.62 mm, p=0.12), minimal lumen diameter (1.98 mm vs. 1.98 mm, p=0.94), and percent diameter stenosis (27.0% vs. 24.7%, p=0.27). In terms of late loss, as with other parameters, no difference was found (0.59 mm vs. 0.50 mm, p=0.10).
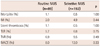
One-year clinical outcomes are presented in Table 4. Mortality rates were around 1% in both groups with no significant difference between them. In addition, the rate of MI was not statistically different between the two groups, which were both less than 5% (2.0% vs. 4.9%, p=0.44). In terms of stent thrombosis, no difference was found in the two groups. Accordingly, the rate of hard end points was not different. Soft end points, such as, TLR and TVR, showed no differences between the two groups (TLR; 5.7% vs. 6.8%; p=0.75, TVR; 6.9% vs. 9.5%; p=0.49). Taken together, the overall MACE (mortality, MI, stent thrombosis, TLR and TVR) during the 1 year follow-up period was not different between the two groups.
The main finding of this study is that there were no significant differences in angiographic and clinical outcomes between the routinely IVUS-guided PCI and selectively IVUS-guided PCI groups.
There have been some studies on the utility of routine IVUS during PCI in the pre-DES era. The results in the pre-DES era were different among the studies. In the OPTICUS study by Mudra et al.,15) a case-control study, they could not support the routine use of ultrasound guidance during PCI, and the results demonstrated that angiography-guided PCI could be performed with comparable clinical outcomes. Likewise, the AVID trial performed as a randomized controlled study failed to show reduction of clinical events for the entire subjects.17) Also, the PRESTO trial failed to demonstrate improved clinical outcomes, although IVUS influenced procedure characteristics.18) On the contrary, the CRUISE study, a multicenter study, demonstrated that TVR at 9 month-follow-up occurred significantly less frequently in the IVUS-guided group.12) Moreover, the TULIP study, which investigated long lesions, showed superior result in the IVUS group over the angiography group in terms of TLR and MACE.13) Choi et al.19) also reported that the use of IVUS guidance during stent implantation was associated with a significant decrease in 6-month TVR without increasing the procedure time, fluoroscopy exposure, contrast volume, or device utilization.19)
In the DES era, Roy et al.16) performed a study on the utility of IVUS during PCI with DES. In their study, IVUS was proven to give rise to less definite stent thrombosis and showed the tendency of less TLR. Therefore the study is thought to raise the possibility that routine IVUS use in the DES era could result in better clinical outcomes compared with angiography-guided PCI. However, this study was not intended to investigate the utility of routine IVUS vs. selective IVUS use in the DES era.
In the present study, we compared two different strategies of IVUS-guidance, routinely vs. selectively IVUS-guided PCI rather than IVUS- vs. angiography-guided PCI, because IVUS-guidance is theoretically more helpful compared to angiographic guidance by assuring good apposition and optimal expansion which are both important factors to determine stent thrombosis or restenosis.
It is certain that IVUS is a useful tool to visualize vessel cross sections, thereby enabling us to confirm underexpansion, malapposition or edge tears. After confirming these unfavorable phenomena, we could perform additional interventions such as post-dilatation or stenting. Ultimately, these processes can improve long term clinical results. In fact, there was less definite stent thrombosis in the IVUS group in the study of Roy et al.16) although the cases were rare. The authors suggested that reduced minimal stent area and stent expansion along with greater residual disease are associated with stent thrombosis after DES implantation. IVUS use may allow the identification of these unfavorable factors and lead to appropriate subsequent treatment.
The remaining question is: which is the optimal strategy for IVUS-guidance, the routine use vs. the selective use? Our results showed that the selective use of IVUS was able to provide as good angiographic and clinical results as the routine use of IVUS during PCI. Therefore, considering our results, an experienced operator's ability to selectively use IVUS may save unnecessary use of IVUS during PCI. The results collectively suggest that adequate, but not absolute, IVUS use can produce good outcomes.
As with clinical outcomes, there have been contradicting studies in the BMS era. The OPTICUS study showed that repeat angiography revealed no significant differences between the IVUS-guided group and angiography-guided group with respect to dichotomous restenosis rate, minimal lumen diameter, and percent diameter stenosis.15) On the contrary, the CRUISE study demonstrated that the IVUS-guided group had a larger minimal lumen diameter by quantitative coronary angiography (QCA) and a larger minimal stent area at 9-month follow-up angiography.12) In addition, the TULIP study reported that at 6 months, MLD in the IVUS group was larger than that in the angiography group.13)
To our knowledge, there has been no report regarding angiographic results judged by QCA in the DES era. Moreover, there have been no studies comparing the two different strategies of IVUS-guidance. In our study, the angiographic data from the 'selective' IVUS-guidance group were comparable to those from the 'routine' IVUS-guidance group. Theoretically, IVUS is believed to produce more favorable angiographic results at follow-up angiography, because it gives us more chances to correct malapposition and underexpansion. However, considering our results, the use of IVUS in need is sufficient to produce results that are comparable to those of absolute IVUS use in the DES era. This might mean that an experienced operator could discriminate the case that is in need of IVUS and perform appropriate additional procedures and produce good results without routine IVUS use. In addition, the potential of DES to reduce the rate of restenosis may contribute to this favorable result of selection IVUS-guidance group.
Coronary intervention is a sophisticated procedure. It has been well established that malapposition and underexpansion is associated with restenosis.20-22) As we know, post-dilatation is a very useful procedure after examination of IVUS to correct malapposition and underexpansion. According to the study of Roy et al., the rates of post-dilatation were 31.0% and 17.7% in the IVUS group and No IVUS group, respectively. Comparing with this result, a post-dilatation rate of about 75% is considered high in the present study. This high post-dilatation rate might have contributed to the comparable outcomes in the selectively IVUS-guided group, diminishing the beneficial effect which may be due to routine IVUS use. Furthermore, this raises a new hypothesis that the routine use of post-dilatation for optimized stent expansion would suffice for good outcome of coronary intervention instead of the routine use of IVUS.
Most previous case-control studies and randomized clinical trials have dealt with both extreme ends, angiography- vs. IVUS-guidance PCI. However, recently, coronary interventionists all agree with the usefulness of IVUS. Thus, many physicians may be pressured to do routine IVUS-guidance. However, in the real world practice, most interventionists probably do not use IVUS in all patients. Instead, they may use IVUS at their discretion, if it is available in their catheterization laboratory. In such situations, our results may provide a helpful message. Given the relatively high percentage of post-dilatation, selective IVUS use would result in adequate clinical and angiographic outcomes. Therefore, when faced with suspicion for underexpansion and malapposition, we can perform post-dilatation without the routine use of IVUS. Furthermore, it is requested to perform a randomized study to determine whether routine post-dilatation is sufficient to obtain good results without IVUS.
Our study has several limitations. First, this study was a nonrandomized retrospective analysis from one center. Therefore, the results and conclusions are subject to the limitations inherent in this type of studies. Second, we collected patients with available QCA data. Therefore, patients who were excluded from the study by this reason could not be analyzed, resulting in the potential introduction of selection bias. Third, the baseline characteristics including gender, dyslipidemia, and vessel territory were different. These may have introduced bias in the analysis. Fourth, for the strategy of selective IVUS, there may be a wide spectrum of IVUS usage percent for different operators, because judgement for underexpansion, malapposition or edge tear is subjective.
Figures and Tables
Acknowledgments
This study was supported by a grant from the Clinical Research Center for Ischemic Heart Disease, Seoul, Republic of Korea (0412-CR02-0704-0001) and a grant from the Innovative Research Institute for Cell Therapy, Seoul National University Hospital (A062260), sponsored by the Ministry of Health, Welfare & Family, Republic of Korea.
References
1. St Goar FG, Pinto FJ, Alderman EL, Fitzgerald PJ, Stadius ML, Popp RL. Intravascular ultrasound imaging of angiographically normal coronary arteries: an in vivo comparison with quantitative angiography. J Am Coll Cardiol. 1991; 18:952–958.
2. Tobis JM, Mallery JA, Gessert J, et al. Intravascular ultrasound cross-sectional arterial imaging before and after balloon angioplasty in vitro. Circulation. 1989; 80:873–882.
3. Baptista J, di Mario C, Escaned J, et al. Intracoronary two-dimensional ultrasound imaging in the assessment of plaque morphologic features and the planning of coronary interventions. Am Heart J. 1995; 129:177–187.
4. Gussenhoven EJ, Essed CE, Lancée CT, et al. Arterial wall characteristics determined by intravascular ultrasound imaging: an in vitro study. J Am Coll Cardiol. 1989; 14:947–952.
5. Tobis JM, Mallery J, Mahon D, et al. Intravascular ultrasound imaging of human coronary arteries in vivo. Analysis of tissue characterizations with comparison to in vitro histological specimens. Circulation. 1991; 83:913–926.
6. Nakamura S, Mahon DJ, Maheswaran B, Gutfinger DE, Colombo A, Tobis JM. An explanation for discrepancy between angiographic and intravascular ultrasound measurements after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1995; 25:633–639.
7. Kastrati A, Dibra A, Mehilli J, et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation. 2006; 113:2293–2300.
8. Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006; 48:2584–2591.
9. Carrozza JP Jr, Kuntz RE, Levine MJ, et al. Angiographic and clinical outcome of intracoronary stenting: immediate and long-term results from a large single-center experience. J Am Coll Cardiol. 1992; 20:328–337.
10. Cavaye DM, White RA. Intravascular ultrasound imaging: development and clinical applications. Int Angiol. 1993; 12:245–255.
11. Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007; 369:667–678.
12. Fitzgerald PJ, Oshima A, Hayase M, et al. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation. 2000; 102:523–530.
13. Oemrawsingh PV, Mintz GS, Schalij MJ, Zwinderman AH, Jukema JW, van der Wall EE. TULIP Study. Thrombocyte activity evaluation and effects of Ultrasound guidance in Long Intracoronary stent Placement. Intravascular ultrasound guidance improves angiographic and clinical outcome of stent implantation for long coronary artery stenoses: final results of a randomized comparison with angiographic guidance (TULIP Study). Circulation. 2003; 107:62–67.
14. Schiele F, Meneveau N, Seronde MF, et al. Medical costs of intravascular ultrasound optimization of stent deployment. Results of the multicenter randomized 'REStenosis after Intravascular ultrasound STenting' (RESIST) study. Int J Cardiovasc Intervent. 2000; 3:207–213.
15. Mudra H, di Mario C, de Jaegere P, et al. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS Study). Circulation. 2001; 104:1343–1349.
16. Roy P, Steinberg DH, Sushinsky SJ, et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J. 2008; 29:1851–1857.
17. Russo RJ, Silva PD, Teirstein PS, et al. A randomized controlled trial of angiography versus intravascular ultrasound-directed bare-metal coronary stent placement (the AVID Trial). Circ Cardiovasc Interv. 2009; 2:113–123.
18. Orford JL, Denktas AE, Williams BA, et al. Routine intravascular ultrasound scanning guidance of coronary stenting is not associated with improved clinical outcomes. Am Heart J. 2004; 148:501–506.
19. Choi JW, Goodreau LM, Davidson CJ. Resource utilization and clinical outcomes of coronary stenting: a comparison of intravascular ultrasound and angiographical guided stent implantation. Am Heart J. 2001; 142:112–118.
20. Rogacka R, Latib A, Colombo A. IVUS-Guided Stent Implantation to Improve Outcome: A Promise Waiting to be Fulfilled. Curr Cardiol Rev. 2009; 5:78–86.
21. Hong MK, Mintz GS, Lee CW, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006; 27:1305–1310.
22. Fujii K, Mintz GS, Kobayashi Y, et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for instent restenosis. Circulation. 2004; 109:1085–1088.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download