Abstract
Background and Objectives
The sinus venosus (SV) is not a well known source of atrial tachycardia (AT), but it can harbor AT during catheter ablation of atrial fibrillation (AF).
Subjects and Methods
A total of 1223 patients who underwent catheter ablation for AF were reviewed. Electrophysiological and electrocardiographic characteristics and outcomes after catheter ablation of AT originating from the SV were investigated.
Results
Ten patients (0.82%) demonstrated AT from the SV (7 males, 53.9±16.0 years, 6 persistent) during ablation of AF. The mean cycle length was 281±73 ms. After pulmonary vein isolation and left atrial ablation, AF converted to AT from the SV during right atrial ablation in 2 patients, by rapid atrial pacing after AF termination in 7 patients, and during isoproterenol infusion in 1 patient. Positive P-waves in inferior leads were shown in most patients (90%). The activation sequence of AT was from proximal to distal in the superior vena cava and high to low in the right atrium, which was similar to that of AT from crista terminalis. Fragmented double potentials were recorded during sinus, and a second discrete potential preceded the onset of P wave by 80±37 ms during AT. Using 4.4±2.7 radiofrequency focal applications, ATs were terminated and became no longer inducible in all. After ablation procedure, two patients showed transient right phrenic nerve palsy. After 19.9±14.8 months, all but 1 patient were free of atrial tachyarrhythmia without complications.
The vast majority of patients convert to atrial tachycardia (AT) upon atrial fibrillation (AF) termination during catheter ablation for AF. As such, ablation of these subsequent organized arrhythmias is an integral part of the procedure to achieve sinus rhythm.1-3) Although catheter ablation can successfully eliminate AT with the use of electroanatomical and/or entrainment mapping with precision, these ATs are sometimes difficult to identify and eliminate. Moreover, the sinus venosus (SV) is not a well known source of AT developed during catheter ablation of AF. The detailed clinical, electrophysiologic, and electrocardiographic characteristics and outcomes of AT originating from the SV during ablation of AF have not been described before. Thus, the aim of this study is to determine the prevalence and characteristics of AT originating from the SV during the ablation of AF; and to evaluate outcomes of these ATs after catheter ablation in a series of patients undergoing catheter ablation for AF.
A total of 1223 consecutive patients who underwent catheter ablation for drug-refractory AF from June 1998 to October 2011 at our institution were screened. Among them, 10 consecutive patients demonstrated AT originating from SV developed during catheter ablation of AF. These 10 patients were considered for the present analysis and constituted the study population. Electrophysiological and electrocardiographic characteristics and outcomes after catheter ablation of AT originating from SV were reviewed.
The local Institutional Review Board approved the study, and written informed consent was obtained from all patients.
Treatment with anti-arrhythmic medication was discontinued at least 5 half-lives before the procedure. Amiodarone was discontinued at least 6 weeks before the ablation procedure. Transesophageal echocardiography was performed to exclude the presence of atrial thrombus within 24 hours prior to the procedure. The ablation procedure was performed under sedation with propofol, while blood pressure and oxygen saturation were monitored. The high right atrium (RA) was mapped with a decapolar catheter (Bard Electrophysiology Inc., Lowell, MA, USA); and the low RA and coronary sinus (CS) were mapped with a duo-decapolar catheter (St. Jude Medical Inc., Minnetonka, MN, USA). A quadripolar catheter was also placed in the superior vena cava (SVC). Intracardiac electrograms were recorded using an electrophysiology system (Prucka CardioLab™ General Electric Health Care System Inc., Milwaukee, WI, USA). After double transseptal puncture, anticoagulation was started with unfractionated heparin, maintaining an activated clotting time between 350 and 400 seconds. We used 3-dimensional (3D) mapping guided geometry (NavX System, St. Jude Medical Inc., St. Paul, MN, USA) for electroanatomical mapping.
All patients initially underwent circumferential antral ablation with electrical pulmonary vein (PV) isolation, while PVs were mapped with the bipolar signals recorded by a 20-pole circular Lasso catheter (Biosense Webster Inc., Diamond Bar, CA, USA). The elimination of the PV potentials was confirmed. Following antral ablation of PVs in persistent AF patients, electrogram-guided ablation for complex fractionated atrial electrograms (CFAEs) at the left atrium (LA) was performed when AF persisted. CFAE was defined by 3D automated software of NavX system as previously demonstrated.4) If AF persisted after LA lesions, radiofrequency (RF) application was continued in the RA, especially at the cavotricuspid isthmus, SVC, crista terminalis, and the RA septum. RF energy was delivered with the target temperature of 48℃ and 25 to 35 W power (Stockert generator, Biosense Webster Inc., Diamond Bar, CA, USA), using a 4-mm open irrigated-tip catheter (Thermocool, Bio-sense Webster, or Duo cool path, St. Jude Medical Inc., St. Paul, MN, USA).
Atrial tachycardia was defined, in accordance with the current consensus statement of a joint expert group, as a regular, supraventricular rhythm with a cycle length ≥200 ms and a consistent atrial activation sequence. Macro-reentry was distinguished from focal tachycardia as described previously.5) Focal AT was defined as an atrial activity originating from a discrete site, centrifugally activating the surrounding tissue and demonstrating features consistent with a focal mechanism. Entrainment mapping was performed to identify the site with less than 20 ms of post-pacing intervals compared to the tachycardia cycle length, which was considered to be in close proximity to the reentrant circuit of AT.6) Any cycle length irregularity or an inconsistent post-pacing interval following entrainment pacing was considered to favor a focal substrate. Focal ATs were firstly mapped by assessing the earliest endocardial activation in relation to P wave onset. RF energy was delivered with a 4 mm-irrigated tip catheter at the same power and temperature settings as used during the AF procedure. During RF catheter ablation, when the AT was not affected at all within 10 seconds, we terminated the RF application, and the catheter was repositioned.
P wave morphology during AT in each of the 12 electrocardiography (ECG) leads was analyzed and classified as positive (+), negative (-), isoelectric, or bi-phasic.
The end points of the procedure were the termination of the AT by RF ablation and the inability to re-induce any atrial tachyarrhythmia by programmed atrial stimulation. The induction test protocol consisted of rapid atrial pacing with 10-second burst pacing in the high RA or CS with pacing cycle length of 150 ms, resulting in a 1 : 1 atrial capture. This test was repeated at least 3 times. AF and AT were considered inducible if they persisted for more than 5 minutes and 10 minutes, respectively.7) If AF or AT became sustained, additional mapping and ablation were performed, aiming at termination of the arrhythmia. If AF had not terminated or was still inducible after ablation of all target sites, sinus rhythm was restored by cardioversion.
Patients were monitored and treated with intravenous heparin overnight, then discharged with a prescription for warfarin, targeting the international normalized ratio ≥2.0. Warfarin was continued until at least 3 months after the procedure. Patients resumed the anti-arrhythmic medications that they had been taking before the procedure. The patients were seen in an outpatient clinic at 1 week and 1, 3, 6, 9, and 12 months after the procedure and then every 6 months thereafter. 12-lead ECG was performed at every visit. A 24-hour holter ECG monitoring analysis was evaluated at 3, 6, and 12 months after the ablation. A detailed history of the patient symptoms suggesting potential AF or AT recurrences was taken. Any episode of AF or AT, including atrial flutter of at least 30 seconds duration that occurred after the blanking period, was classified as a recurrence.8) At the 3-month visit, treatment with anti-arrhythmic agents was discontinued if there was no evidence of recurrence. Warfarin therapy was discontinued, and aspirin was substituted if monitoring did not reveal any recurrence of AF and/or AT.
Ten patients (0.82%) showed AT from SV (7 males, 53.9±16.0 years, 6 persistent and 4 paroxysmal), developed during ablation of AF. The baseline clinical characteristics are summarized in Table 1. The mean duration from the first diagnosis of AF to ablation was 5.9±3.1 (range 2-10) years. The LA diameter was 40.1±6.0 mm in the parasternal window, and left ventricular ejection fraction was 51.7±7.0%. Patients No. 2, 7, 8, and 10 had hypertensive heart disease. Patients No. 2, 4, 6, 8, 9, and 10 had received prior catheter ablation for AF, among them patient 6 and 9 had undergone two procedures of catheter ablation for AF before.
Electrophysiologic characteristics in each patient are summarized in Table 2. AT was sustained in 9 and non-sustained in 1 patient. The mean cycle length was 281±73 ms. After PV isolation and LA ablation, patients No. 2 and 3 spontaneously developed AT from SV during RA ablation; patients No. 1, 4, 5, 7, 8, 9 and 10 developed AT by rapid atrial pacing; and patient No. 6 showed AT during isoproterenol infusion. The activation sequence of AT was from proximal to distal in SVC and high to low at RA in all patients (Fig. 1). The activation sequence of CS was proximal to distal in all, except three patients who received linear peri-mitral ablation with a bidirectional block before developing AT from SV. Fragmented double potentials were recorded during sinus, and a second discrete potential preceded 80±37 ms before the onset of P wave during AT (Figs. 1 and 2). Patient No. 4 revealed that the potential at SV to other areas was 2 : 1 conduction (Fig. 3). RA activation mapping using 3D was performed in patient No. 2 and 3. Both patients revealed that the earliest activation site was SV (Fig. 4). During atrial overdrive pacing at SV, the difference between post-pacing interval and tachycardia cycle length was less than 10 ms in all patients. Three patients received RF application at the crista terminalis (mean: 3.7±2.5; range: 1-6) for the target of AT before ablation at SV, and AT sustained. Patients No. 4, 5, 9, and 10 showed different morphology of AT originating from the RA septum, LA anterior wall, crista terminalis, and CS ostium, respectively, before developing AT from SV.
The results of a comparison of P wave morphology during AT are given in Table 3. All but one patient showed positive P waves in inferior leads (II, III and aVF). Four patients showed negative P waves in the V 1 lead, and 2 patients showed a positive-negative bi-phasic wave. Only 3 patients (30.0 %) exhibited positive P wave in aVL.
Using focal RF applications in those regions with mean frequency of 4.4±2.7, ATs were successfully terminated, as shown in Fig. 5. A representative example of the fluoroscopic and three-dimensional images of the ablation site is shown in Fig. 6. All the patients revealed that the precise location of ablation was the superior part of the SV. Focal RF ablation was successful in eliminating AT, and atrial tachyarrhythmias were no longer inducible in any patient.
Two patients showed transient right phrenic nerve palsy (PNP), which induced dyspnea and coughing after the ablation procedure. The PNP was transient in both patients, and the elevated right diaphragm was fully resolved in the chest roentgenogram 6 months after the ablation procedure. No patient showed sinus node dysfunction after ablation. There were no serious complications such as perforation, thromboembolism, or atrioesophageal fistula related to the ablation procedure. After a mean follow-up of 19.9±14.8 months, AT recurred in only 1 patient; this patient underwent a repeated ablation procedure. Critical termination site of AT was left inferior PV in this patient. AF recurred in 3 patients; cardioversion restored sinus rhythm and well controlled with anti-arrhythmic drugs; in 2 patients and 1 patient remained in AF.
Other than this one patient, all patients were free from any arrhythmia recurrences thereafter. Six patients did not require anti-arrhythmic drugs.
This study demonstrates that the incidence of AT from SV developed during AF ablation is as rare as 0.82% among patients undergoing AF ablation. The activation sequence of AT was from proximal to distal in SVC and CS, and from high to low at RA in all patients, which was similar to AT from crista terminalis. In electrocardiographic analysis, all except one patient showed positive P waves in inferior leads, and 6 patients (60.0%) showed negative or positive-negative bi-phasic P wave in lead V 1. Focal application of RF was successful in all patients, and most patients remained arrhythmia-free at follow-up. Transient right PNP developed in two patients (20.0%).
Atrial tachycardias often occur immediately after termination of AF by RF application or during follow-up. AT during AF ablation has been reported to occur in 10-30% of patients with paroxysmal and persistent AF.3)9)10) The development commonly originates from around the PVs related to the ablation procedure and arrhythmogeneicity of the PV antrum.11) Additionally, the superior base of the left atrial appendage, inside CS, crista terminalis, and SVC were reported as the origins of ATs.11)12) The SV is a smooth-walled structure and has no grossly distinguishable structural barriers, but there are abrupt changes in the thickness and orientation of the muscle fibers, as well as in the collagen content.13) Such microscopic discontinuities have been suggested to provide a basis for rate-dependent conduction blocks during transverse atrial activation.14) Among atrial arrhythmia patients, SV was sometimes observed as a low voltage zone forming the borders of the slow conduction isthmus for the critical reentrant circuit.15) SV is anatomically in close proximity to the crista terminalis anteriorly and the septum at the insertion of Bachmann's bundle and to the SVC superiorly. Detailed mapping is required to differentiate AT from these neighboring structures. The SV has not previously been studied as the focal origin of AT during ablation of AF. To our knowledge, this study is the first study evaluating AT from SV developed during ablation of AF.
Mapping ATs during ablation of AF is sometimes challenging because of coexisting multiple tachycardias and frequent and subtle changes in the activation sequence due to previous ablation lines. Detailed entrainment mapping has the potential to gain information on the arrhythmia mechanism, for example, by observing inconsistent post-pacing responses at a single pacing site in a case of focal AT. This is particularly useful when focal AT appears to have stable cycle lengths. Furthermore, the length and range of variation of the post-pacing interval provides information about the distance of the pacing site to the AT reentrant circuit or focus.6) Recently a new mapping algorithm for subsequent ATs occurring immediately after AF termination was reported.16) In that approach, the degree of AT cycle length stability was assessed as the first step, followed by construction of a detailed activation map to delineate the propagation direction of the macro-reentrant AT. Entrainment mapping at the deduced critical site of the focal AT was used to confirm the diagnosis. With this deductive mapping algorithm, the accuracy was 97% with a relatively brief mapping time of 11 minutes per AT.16) In our study, the combination of activation mapping and detailed entrainment was used. We first suspected the SV as the origin of focal AT by activation sequence, from proximal to distal in SVC and CS and from high to low at the crista terminalis and RA and centrifugal activation from SV. The diagnosis was confirmed by entrainment pacing showing the shortest post-pacing interval at SV and centrifugal mode of post-pacing interval response. Concealed entrainment pacing at SV was performed in 5 patients, and all patients showed less than 10 ms difference between post-pacing interval and tachycardia cycle length. Although these pacing maneuvers have the potential to terminate or convert the interrogated AT, such counterproductive entrainment is rarely observed in clinically stable ATs.16)
Prediction of foci using the algorithm of the P wave is useful for catheter ablation of ATs with structurally normal atria. Several studies have demonstrated that P wave morphology in lead aVL or V 1 could distinguish between AT foci in the RA and those in the LA, and that the polarity of the P wave in the inferior leads was helpful in predicting whether AT foci were located in the superior or inferior portion of the atrium.17-19) However, the accuracy of P wave morphology is limited when there is significant atrial disease, which is most commonly seen after catheter ablation for AF. Furthermore, the accuracy in predicting AT from SV using the algorithm of a positive P wave in aVL, negative in V 1, and positive in the inferior leads had not been examined before. In the current study, most patients (90.0%) showed a positive P wave in the inferior leads, suggesting origination from a superior portion of the atrium. But it is difficult to localize the site precisely in the superior portion of atrium and to differentiate with AT especially from crista terminalis by electrocardiographic finding only.20) Substantial ratio of patients (60.0%) revealed negative or positive-negative bi-phasic P wave in lead V 1, but only 30.0% showed a positive P wave in aVL. These results suggest that some caution should be exercised in the interpretation. An integrative analysis of activation and entrainment mapping in conjunction with ECG are warranted for efficient localization of these ATs.
In a previous report, right PNP developed during ablation of the right superior PV or SVC disconnection. Ablation of the postero-septal part of the SVC was likely to be associated with right PNP.21) Also, an anatomical study revealed that the course of the right phrenic nerve runs over part of the junction between the SVC and RA, which is close to the SV.22) Therefore, right PNP may occur during catheter ablation at the site of SV of the RA. Two patients developed transient right PNP with full recovery within 6 months in our study. Pacing at maximum output should be considered prior to applying RF at the SV to avoid such complication.
This is a retrospective study which has some known limitations, with the small sample size of the study group being the major limitation. Total incidence of AT developed during AF ablation was not evaluated. No comparisons were made with other sources of AT during ablation of AF, because the incidence of AT from SV was too low. Site of ablation, SV was only guided by fluoroscopic anatomy. In addition, the clinical significance of AT originating SV during AF ablation has not been determined as yet; further observation is needed on whether these tachycardias resolve spontaneously with time or sustain. Previously, a substantial number of patients who developed macro-reentrant AT during LA circumferential ablation for AF showed spontaneous resolution.2) Lastly, the patients were followed for a relatively short duration, constituting a limitation with respect to AT developing over the long-term.
Sinus venosus needs to be considered as the origin of secondary AT during catheter ablation of AF, although it is very rare. Electrocardiographic and electrophysiologic findings are helpful in identifying the origin of AT and in avoiding unnecessary multiple ablation lines. Although catheter ablation of this tachycardia may be challenging, it is highly effective when using focal ablation and has an excellent success rate in most patients. Special caution is needed to avoid right PNP in such cases.
Figures and Tables
Fig. 1
A representative example of activation sequence during atrial tachycardia from SV in patient No. 3. Activation sequence was proximal (P) to distal (D) in the SVC and CS and from high to low at the RA. The earliest potential at the distal ablation catheter (ABL) preceded the onset of P wave at surface ECG by 97 ms. CS: coronary sinus, HRA: high right atrium, SV: sinus venosus, SVC: superior vena cava, ECG: electrocardiograpy.
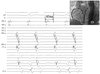
Fig. 2
Double potentials were recorded during sinus (A), and a second discrete potential (*) preceded the onset of P wave during atrial tachycardia by mean of 80±37 ms (B). ABL: ablation catheter, HRA: high right atrium, CS: coronary sinus, AT: atrial tachycardia.

Fig. 3
Patient No. 4 showed 2 : 1 conduction potentials from the SV (*) to other areas. SV: sinus venosus, ABL: ablation catheter, HRA: high right atrium, CS: coronary sinus.

Fig. 4
RA activation mapping revealed that the earliest activation site (white arrow) was SV. RA: right atrium, SV: sinus venosus.

Fig. 5
Effects of radiofrequency ablation. Radiofrequency energy delivery at the SV slowed the tachycardia cycle length from 345 to 355 ms (A) and finally resulted in sinus rhythm within 56 seconds (B). There were no inducible tachycardias thereafter. SV: sinus venosus, RFCA: radiofrequency catheter ablation, ABL: ablation catheter, HRA: high right atrium, CS: coronary sinus.
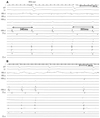
Fig. 6
A representative example of fluoroscopic and three-dimensional images of the ablation site, SV (white arrow). Right-anterior oblique projection view 35° (A); left-anterior oblique projection view 35° (B); and three-dimensional images, white arrow and yellow dot indicates SV where AT terminated during RF application (C). ABL: ablation catheter, CS: coronary sinus, HRA: high right atrium, RF: radiofrequency, SV: sinus venosus, SVC: superior vena cava, AT: atrial tachycardia.

Table 2
Electrophysiologic characteristics of the study patients

AT: atrial tachycardia, CS: coronary sinus, D: distal, EA-P: interval from earliest potential at the successful ablation site to P-wave onset, H: high, L: low, N/E: not evaluated, P: proximal, RA: right atrium, RAP: rapid atrial pacing, RF: radiofrequency, SVC: superior vena cava, TCL: tachycardia cycle length
Acknowledgments
We presented our study in part as an oral abstract in the 4th Asian Pacific Heart Rhythm Society 2011 in Fukuoka, Japan.
References
1. Mesas CE, Pappone C, Lang CC, et al. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: electroanatomic characterization and treatment. J Am Coll Cardiol. 2004. 44:1071–1079.
2. Chugh A, Oral H, Lemola K, et al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm. 2005. 2:464–471.
3. Chae S, Oral H, Good E, et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol. 2007. 50:1781–1787.
4. Scherr D, Dalal D, Cheema A, et al. Automated detection and characterization of complex fractionated atrial electrograms in human left atrium during atrial fibrillation. Heart Rhythm. 2007. 4:1013–1020.
5. Saoudi N, Cosío F, Waldo A, et al. A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases; a Statement from a Joint Expert Group from The Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 2001. 22:1162–1182.
6. Mohamed U, Skanes AC, Gula LJ, et al. A novel pacing maneuver to localize focal atrial tachycardia. J Cardiovasc Electrophysiol. 2007. 18:1–6.
7. Jaïs P, Hocini M, Sanders P, et al. Long-term evaluation of atrial fibrillation ablation guided by noninducibility. Heart Rhythm. 2006. 3:140–145.
8. Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007. 4:816–861.
9. Nam GB, Jin ES, Choi H, et al. Mechanism of regular atrial tachyarrhythmias during combined pulomonary vein isolation and complex fractionated electrogram ablation in patients with atrial fibrillation. Circ J. 2010. 74:434–441.
10. Rostock T, Drewitz I, Steven D, et al. Characterization, mapping, and catheter ablation of recurrent atrial tachycardias after stepwise ablation of long-lasting persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2010. 3:160–169.
11. Satomi K. Electrophysiological characteristics of atrial tachycardia after pulmonary vein isolation of atrial fibrillation. Circ J. 2010. 74:1051–1058.
12. Haïssaguerre M, Hocini M, Sanders P, et al. Catheter ablation of longlasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005. 16:1138–1147.
13. Gonzalez MD, Erga KS, Rivera J, et al. Rate-dependent block in the sinus venosa of the swine heart during transverse right atrial activation: correlation between electrophysiologic and anatomic findings. J Cardiovasc Electrophysiol. 2005. 16:193–200.
14. Harada M, Osaka T, Yokoyama E, Takemoto Y, Ito A, Kodama I. Action potential characteristics in the sinus venosa of patients with and without atrial flutter. Circ J. 2009. 73:647–653.
15. Lin YJ, Higa S, Tai CT, et al. Role of the right atrial substrate in different types of atrial arrhythmias. Heart Rhythm. 2009. 6:592–598.
16. Jaïs P, Matsuo S, Knecht S, et al. A deductive mapping strategy for atrial tachycardia following atrial fibrillation ablation: importance of localized reentry. J Cardiovasc Electrophysiol. 2009. 20:480–491.
17. Kistler PM, Roberts-Thomson KC, Haqqani HM, et al. P-wave morphology in focal atrial tachycardia: development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol. 2006. 48:1010–1017.
18. Tang CW, Scheinman MM, Van Hare GF, et al. Use of P wave configuration during atrial tachycardia to predict site of origin. J Am Coll Cardiol. 1995. 26:1315–1324.
19. Kistler PM, Kalman JM. Locating focal atrial tachycardias from P-wave morphology. Heart Rhythm. 2005. 2:561–564.
20. Qian ZY, Hou XF, Xu DJ, et al. An algorithm to predict the site of origin of focal atrial tachycardia. Pacing Clin Electrophysiol. 2011. 34:414–421.
21. Sacher F, Monahan KH, Thomas SP, et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol. 2006. 47:2498–2503.
22. Sánchez-Quintana D, Cabrera JA, Climent V, Farré J, Weiglein A, Ho SY. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J Cardiovasc Electrophysiol. 2005. 16:309–313.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download