Abstract
There has been a dramatic increase in the number and type of procedures performed in the field of cardiac intervention in the past decade. Percutaneous intervention is becoming an increasingly recognized modality for the management of prosthetic paravalvular leakages (PVLs) in severely symptomatic non-surgical candidates. Herein, we report our experience of percutaneous closure using the Amplatzer duct occluder for a PVL in a patient who underwent tricuspid valve replacement.
The first double-umbrella device developed for intracardiac use, which was introduced in 1976, received little attention.1) However, since the development of the double-umbrella device for transcatheter closure of patent ductus arteriosus (PDA) by Rashkind et al.2) in 1979, percutaneous transcatheter closure techniques have come into routine application in the management of atrial septal defects (ASDs), PDA, and other pathologic cardiac and vascular communications. These techniques have also been applied to paravalvular leakages (PVLs).3)4)
Paravalvular leakages most commonly result from the rupture of one or more sutures securing the prosthesis to the valve annulus, and 60% of patients with PVLs are diagnosed within the first year after valve replacement.5)6) Clinical manifestations include reduced net cardiac output, transfusion-dependent hemolytic anemia, and congestive heart failure.
Transcatheter percutaneous closure of PVLs may be offered to patients with high operation risks. This technology has been mostly applied to PVLs via a transvenous, transseptal, and antegrade approach. In this report, we describe what we believe to be a proper percutaneous solution for tricuspid PVL.
A 51-year-old woman was diagnosed with ASD at the age of 10 years. However, her disease had not been clinically managed until she presented with cough and palpitation at the age of 37 years. At that time, a large secundum ASD (30×20 mm), rheumatic mitral valve regurgitation of grade 2/4, and tricuspid valve regurgitation of grade 2/4 by incomplete coaptation were identified by transthoracic echocardiography (TTE). Electrocardiography showed atrial fibrillation. Cardiac catheterization revealed a right ventricular end diastolic pressure of 5 mm Hg, left ventricular end diastolic pressure of 5 mm Hg, and mean pulmonary artery pressure of 20 mm Hg, respectively. Since both ventricular functions were good without any evidence of pulmonary hypertension, ASD closure was performed using a Bovine pericardial patch at 37 years of age. TTE after ASD closure showed that the pressure gradient of tricuspid regurgitation of grade 2-3/4 was 33 mm Hg, the right ventricle and the right atrium were enlarged, and both ventricular functions were normal.
However, as time passed, regurgitation flow of the tricuspid valve had progressed and valvular annuluses had been dilated by atrial fibrillation. Additionally, there were degenerative valve changes and occurrence of annular dilatation due to valve regurgitation itself. Besides, by rheumatic changes and atrial fibrillation, regurgitation flow of the mitral valve had also progressed. At ten years of age after ASD closure, she suffered from recurrent respiratory infection and decreased exercise capacity. TTE revealed an enlarged right ventricle which was also severely dysfunctional, tricuspid valve regurgitation of grade 4/4, and annular dilatation. In addition, the enlarged left ventricle had mitral regurgitation of grade 2/4 as well as annular dilatation. Hence, we performed tricuspid valve replacement (TVR) by 29 mm perimounted Carpentier-Edwards (Edwards Lifesciences, Irvine, CA, USA), mitral valve repair, mitral annuloplasty with a 28 mm Carpentier-Edwards ring (Edwards Lifesciences, Irvine, CA, USA), and Maze III operation with cryoablation. During the post-operative period, she had right ventricular dysfunction that required extracorporeal membrane oxygenation but the patient was weaned off on day 10 after the cardiac operation. TTE demonstrated a well functioning tricuspid valve and mild tricuspid regurgitation in the central and paravalvular areas.
She has been admitted to the hospital several times after TVR due to recurrent heart failure, respiratory infections, and pleural effusions. Five years after the valve replacement operation, TTE showed a moderate amount of tricuspid valve regurgitation via PVL with a dilated inferior vena cava and decreased right ventriclular function (Fig. 1). Due to ventricular dysfunction and a history of post-operative mechanical support, she refused surgery to correct her condition at that time. Thus, we opted for percutaneous device closure of the PVL.
Percutaneous closure was performed via an antegrade femoral approach under general anaesthesia with trans-esophageal echocardiogram (TEE) and fluoroscopic guidance. The PVL was located in the medial side of the tricuspid valve. The defect was selected by an angled hydrophilic guidewire of 0.035 inches (Glide wire, Terumo Inc., Japan) and the diameter of the leak measured by right ventriculography was 7.8 mm. Therefore, we chose the Amplatzer duct occluder (ADO) for deployment because of its "double flange" configuration. An ADO measuring 12×10 mm was positioned in the paravalvular defect and in TEE, mild tricuspid valve regurgitation was found owing to the elliptical nature of the defect (Fig. 2). By day 5, tricuspid valve regurgitation became mild, likely due to the seating of the device and edema of the surrounding tissues. Repeat TTE in the third month post-procedure showed the device to be well seated, and both ventricles demonstrated good function. Early complications, including stroke, embolism, arrhythmia and infection, were absent. After 14 months, the patient showed improvement in her respiratory symptoms and the New York Heart Association function class returned from IV to II.
Paravalvular leakage is a well-recognized complication of prosthetic cardiac valves with frequency ranging from 3 to 7% in long-term studies.7)8) This condition is most commonly caused by suture dehiscence between the sewing ring and the valve annulus.9) This process may be precipitated by annular calcification, inherent tissue friability, and infection, or could be secondary to surgical technique.10) In the early period following valve replacement surgery, the occurrence of PVL has been reported to be as high as 47.6%.9) Most PVLs are benign, and only 1-5% of replaced valves are presented with clinically significant regurgitation.11)12)
Medical therapy is the treatment of choice for small leaks. Repeat surgery remains the gold standard for significant mitral regurgitation, particularly when it is associated with other associated symptoms, heart failure, or functional decline.12) Symptomatic hemolytic anemia from regurgitation requiring transfusion is another indication for intervention. However, reoperation for the purpose of PVL repair causes significant morbidity, and is associated with a high rate of perioperative mortality (6-15%).13)
In 1992, Hourihan et al.14) reported the first successful percutaneous repair in three patients with aortic PVL using the Rashkind-Cuaso double umbrella. Percutaneous closure of PVL is a challenging task, but may be feasible in highly selected patients. The procedure may be the treatment of choice in certain individuals whose symptoms are not adequately palliated by medical therapy and who are at high surgical risk.15) Percutaneous closure of PVL is an evolving therapeutic option that offers an alternative to traditional surgical closure for non-surgical candidates. However, in Korea, there are only few cases of percutaneous closure of prosthetic paravalvular leaks.
A transcatheter device specifically designed for PVL repair has not yet been developed, thus a selection is made from an armamentarium of devices designed for other purposes, none of which is approved for this particular use.15) Choosing a device is not easy in most cases because PVLs are commonly elliptical in shape. Early reports have described the use of detachable coils or "clamshell" type devices. Recently, Amplatzer occluders have become more widely used, including those designed primarily for ASDs, muscular ventricular septal defects, or PDA occlusions (AGA Medical Corporation, Golden Valley, MN, USA).16) The ADO was used in the present case as well. The choice and availability of the device depends on the specific size and morphology of the PVL as well as proximity to the adjacent valve leaflets. After percutaneous device closure, many patients may experience slight residual regurgitation due to to the elliptical morphology of PVL. Therefore, over-sizing of the device has several potential advantages. However, the possibility of interference with prosthetic valve leaflets must be identified by echocardiography and may require a change of device.15)
Although rarely reported, there are many potential complications that include the usual complications associated with general anesthesia, TEE, vascular access, and trans-septal puncture. Embolization of thrombus, air, atheroma, or valve material could result in ischemic complications such as stroke. Aggressive attempts to force a delivery catheter through a defect could cause further valve dehiscence. Of course, occurrence of infection remains a matter of concern, as with any other implanted device. For example, deployed devices may interfere with valve leaflets. Device embolization may occur, and this risk is greatest during attempted implantation of a second device when the previously placed device is dislodged. For this reason, it may be advisable to wait several weeks for fibrosis to occur for anchoring of the device before inserting a second one.15)
This intervention is indicated for severely symptomatic patients with a high risk for surgical correction, and it is becoming an established practice worldwide. Still, the long-term efficacy of percutaneous closure remains to be demonstrated. The development of defect-specific devices and techniques is likely to extend the application of this approach and minimize current limitations.17) Herein, we report a case of percutaneous closure which was managed using the ADO for PVL in a patient who underwent TVR.
Figures and Tables
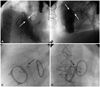
Fig. 2
White arrows represent a paravalvular leakage of the prosthetic tricuspid valve in the left anterior oblique view (A) and the lateral view (B). The Amplatzer duct occluder was positioned in the paravalvular defect. Radio-opaque markers of the Amplatzer duct occluder are noted in the anteroposterior view (C) and the lateral view (black arrows) (D).
References
1. King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA. 1976. 235:2506–2509.
2. Rashkind WJ, Mullins CE, Hellenbrand WE, Tait MA. Nonsurgical closure of patent ductus arteriosus: clinical application of the Rashkind PDA Occluder System. Circulation. 1987. 75:583–592.
3. Piéchaud JF. Percutaneous closure of mitral paravalvular leak. J Interv Cardiol. 2003. 16:153–155.
4. Moscucci M, Deeb GM, Bach D, Eagle KA, Williams DM. Coil embolization of a periprosthetic mitral valve leak associated with severe hemolytic anemia. Circulation. 2001. 104:E85–E86.
5. Hein R, Wunderlich N, Robertson G, Wilson N, Sievert H. Catheter closure of paravalvular leak. EuroIntervention. 2006. 2:318–325.
6. Orszulak TA, Schaff HV, Danielson GK, Pluth JR, Puga FJ, Piehler JM. Results of reoperation for periprosthetic leakage. Ann Thorac Surg. 1983. 35:584–589.
7. Dávila-Román VG, Waggoner AD, Kennard ED, et al. Prevalence and severity of paravalvular regurgitation in the Artificial Valve Endocarditis Reduction Trial (AVERT) echocardiography study. J Am Coll Cardiol. 2004. 44:1467–1472.
8. Sorajja P, Cabalka AK, Hagler DJ, et al. Successful percutaneous repair of perivalvular prosthetic regurgitation. Catheter Cardiovasc Interv. 2007. 70:815–823.
9. Rallidis LS, Moyssakis IE, Ikonomidis I, Nihoyannopoulos P. Natural history of early aortic paraprosthetic regurgitation: a five-year follow-up. Am Heart J. 1999. 138(2 Pt 1):351–357.
10. Dhasmana JP, Blackstone EH, Kirklin JW, Kouchoukos NT. Factors associated with periprosthetic leakage following primary mitral valve replacement: with special consideration of the suture technique. Ann Thorac Surg. 1983. 35:170–178.
11. Bhindi R, Bull S, Schrale RG, Wilson N, Ormerod OJ. Surgery Insight: percutaneous treatment of prosthetic paravalvular leaks. Nat Clin Pract Cardiovasc Med. 2008. 5:140–147.
12. Lasorda DM, Mohsin JC. Percutaneous closure of perivalvular mitral regurgitation with an Amplatzer occluder device in a patient with both prosthetic mitral and aortic valves. J Interv Cardiol. 2008. 21:190–195.
13. Echevarria JR, Bernal JM, Rabasa JM, Morales D, Revilla Y, Revuelta JM. Reoperation for bioprosthetic valve dysfunction. A decade of clinical experience. Eur J Cardiothorac Surg. 1991. 5:523–526. discussion 527.
14. Hourihan M, Perry SB, Mandell VS, et al. Transcatheter umbrella closure of valvular and paravalvular leaks. J Am Coll Cardiol. 1992. 20:1371–1377.
15. Pate GE, Thompson CR, Munt BI, Webb JG. Techniques for percutaneous closure of prosthetic paravalvular leaks. Catheter Cardiovasc Interv. 2006. 67:158–166.
16. Kort HW, Sharkey AM, Balzer DT. Novel use of the Amplatzer duct occluder to close perivalvar leak involving a prosthetic mitral valve. Catheter Cardiovasc Interv. 2004. 61:548–551.
17. Fanning JP, Cox SV, Scalia GM. Percutaneous closure of an aortic prosthetic paravalvar leak: an Australian first. Heart Lung Circ. 2012. 21:174–177.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download