Abstract
A 41-year-old male was presented with drug-resistant supraventricular tachycardia. Electrophysiological study confirmed that the supraventricular tachycardia was caused by dual atrioventricular nodal pathways and a left lateral accessory pathway (AP). The left lateral AP was resistant to traditional endocardial ablation, but was successfully eliminated by radiofrequency ablation via the intracoronary sinus approach.
Supraventricular tachycardia can be caused by diverse aberrant pathways. Dual atrioventricular (AV) nodal physiology, responsible for atrioventricular nodal reentrant tachycardia (AVNRT), has been reported in up to 12 percent of patients with the Wolff-Parkinson-White syndrome. However, supraventricular tachycardia, due to coexisting dual AV nodal pathways and an accessory pathway (AP) in a single patient, is very rare.
We report a patient with supraventricular tachycardia, which was attributed to AVNRT due to dual AV nodal pathways and atrioventricular reentrant tachycardia (AVRT) due to a left lateral AP. Furthermore, a radiofrequency (RF) ablation of the left lateral AP was not successful through a conventional endocardial approach, but it was successful through an intracoronary sinus approach.
A 41-year-old man had a 5-year history of palpitations with exertion, due to a paroxysmal supraventricular tachycardia. The paroxysmal supraventricular tachycardia was intractable to medical treatment, and he was referred for electrophysiological study and RF ablation. The documented electrocardiography of supraventricular tachycardia revealed a heart rate of 168 bpm and no definitive P waves. After obtaining an informed consent, all antiarrhythmic medications were discontinued, and an electrophysiological study was performed. Under local anesthesia and using sterile technique, two quadripolar electrode catheters (St. Jude Medical, Inc., Minnetonka, MN, USA) were positioned to record the activity of the His bundle and right ventricular (RV) apex. The high right atrium (RA), low RA, and coronary sinus (CS) were mapped with a deflectable duo-decapolar catheter (St. Jude Medical, Inc., St. Paul, MN, USA), inserted via the left femoral vein. Intracardiac electrograms were recorded using a Prucka CardioLab™ electrophysiology system (General Electric Health Care System, Inc., Milwaukee, WI, USA).
The retrograde conduction pattern was eccentric and the earliest retrograde atrial activation was recorded in the distal CS during RV pacing (Fig. 1A). A narrow QRS tachycardia was repeatedly inducible with programmed atrial and ventricular stimulation (Fig. 1B). The mechanism of tachycardia was confirmed to be AVRT, based on the advancement of atrial activation, induced by the ventricular extra-stimulation at the time of the refractoriness.
Ablation of the left lateral AP, during a sinus rhythm, was first attempted via a transseptal approach. RF energy was delivered to this site at a temperature of 55℃, but it did fail to eliminate the bypass tract. Despite the repeated RF applications around the left lateral region, the tachycardia remained inducible with programmed atrial stimulation. Extensive RF applications from the left lateral to the posterolateral region were ineffective, as well. After injection of the contrast medium under fluoroscopy in left anterior oblique 45° and right anterior oblique 30° projections, the ablation catheter was positioned in the intra CS (Fig. 2A and B). RF energy delivered at this site, using a maximum power of 30 Watts (Fig. 2C) and a maximum electrode-tissue interface temperature of 50℃ eliminated the retrograde conduction over the AP (Fig. 2D).
Upon programmed atrial stimulation, atypical AVNRT was initiated with antegrade conduction through the fast pathway and retrograde conduction through the slow pathway (fast-slow type) (Fig. 3). The induced tachycardia had a tachycardia cycle length (TCL) of 340 msec, a short AH (140 msec) interval, a long HA interval (210 msec), AH/HA ratio <1, and a long VA interval, over 60 msec (185 msec). Single ventricular extra stimuli with a coupling interval of 360 msec were unable to reset the tachycardia. Following a cessation of ventricular entrainment pacing, the tachycardia resumes with a V-A-V response, and the values of post-pacing interval minus TCL were greater than 115 msec (Fig. 4). Thus, a fast-slow type AVNRT was confirmed. The tachycardia was reproducibly induced upon a decremental atrial pacing and terminated with atrial overdrive pacing. A 7-Fr, 4-mm-tip deflectable ablation catheter (Boston Scientific Corp., Natick, MA, USA) was positioned at the roof of the CS ostium, and slow pathway ablation was attempted in the inferior region of the Koch's triangle, where the local A/V was <0.5. An accelerated junctional rhythm was immediately observed and AVNRT could not be induced with either decremental atrial pacing or programmed atrial stimulation. A post-RF ablation electrophysiology study with isoproterenol did not induce any supraventricular tachycardia. The patient remained asymptomatic three years later.
This case describes a patient who had dual AV nodal pathways and resistant AP localized in the left lateral area. We performed catheter ablation of coexisting AP and a slow pathway. The clinically documented supraventricular tachycardia was AVRT with anterograde conduction over the normal AV conduction system, and retrograde conduction via the left lateral AP. AVNRT was subsequently induced by programmed atrial pacing after the elimination of AP and the slow pathway ablated.
The AP may coexist with dual AV nodal pathways. The dual AV nodal physiology can be observed in more than 10% of patients with Wolff-Parkinson-White syndrome.1) However, supraventricular tachycardia, due to coexisting AP and dual AV nodal pathways in a single patient, is less common. In our patient, the dual AV nodal pathways were unmasked after successful elimination of AP. The slow pathway could be documented and ablated after abolishing the conduction over the AP. The current case suggests the importance of investigating another mechanism of supraventricular tachycardia, even after a successful ablation of the AP.
Another instructive finding in the current case was the successful elimination of the left lateral AP, through the intra CS approach. The left lateral AP was resistant to a conventional endocardial RF ablation via a transseptal approach. Left-sided AV APs, the anatomical substrates for the AVRT composed of muscular bundles, usually course through the AV fat pad between the CS and the annulus fibrosus and hug the hinge point of the mural leaflet of the mitral valve, which can be mapped and ablated from the left atrium, left ventricle, or CS.2)3) Ablation of left free wall AP requires that the tip of the ablation catheter be positioned at a target site along either the atrial or ventricular surface of the mitral annulus. These pathways can be approached from the left cardiac chambers via a transseptal or transaortic approach. Earlier studies reported that the successful rates of endocardial ablation of AP were 87-99%.4)5) There may be a variety of reasons for a lengthy or ablation failure of AP. For example, epicardial insertion of the AP is an important cause of difficult endocardial AP ablation, particularly in the left lateral anatomic location. Haissaguerre et al.6) reported a successful epicardial catheter ablation of the left lateral AP, through the CS, when endocardial approaches were unsuccessful. As in this case, RF ablation via inside the CS would be an effective alternative approach if AP is resistant to the traditional endocardial RF ablation.
Figures and Tables
 | Fig. 1Simultaneous recordings for surface leads I, aVF, V1 and intracardiac electrograms from high right atrium, proximal and distal coronary sinus, His bundle, and right ventricular apex. Earliest atrial (A) activation during right ventricular (V) pacing (A) or spontaneous supraventricular tachycardia induction are recorded from the CS1, 2 electrodes (B). The atrial activation sequence is eccentric and earliest at the distal coronary sinus. HIS: His bundle, RVA: right ventricular apex, HRA: high right atrium, CS: coronary sinus, ABL: ablation. |
 | Fig. 2Ablation of resistant left lateral accessory pathway via the intracoronary sinus approach. A and B: the fluoroscopic images, right anterior oblique and left anterior oblique, showing the position of ablation catheter at distal coronary sinus through intracoronary sinus. C: the local electrogram from distal ABL catheter during right ventricle (RV) pacing has the same ventricular (V) and atrial (A) timing compared to that of proximal CS catheter. D: after radiofrequency energy (RF) application at this point, the electrogram shows the retrograde conduction through HIS during RV pacing. HIS: His bundle, RVA: right ventricular apex, HRA: high right atrium, CS: coronary sinus, ABL: ablation. |
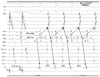 | Fig. 3Initiation of atypical atrioventricular nodal reentrant tachycardia (AVNRT) with programmed atrial stimulation (550/280 msec); AH jump and the induction of atypical AVNRT with antegrade conduction (downward arrow) by fast pathway and retrograde conduction (upward arrow) by slow pathway. The tachycardia cycle length is 342 msec, AH interval is 140 msec, HA interval is 210 msec, AH/HA ratio <1 and VA interval is 185 msec. HRA: high right atrium, HIS: His bundle, ABL: ablation, CS: coronary sinus, RV: right ventricular. |
 | Fig. 4Ablation of atypical atrioventricular nodal reentrant tachycardia. A: delivery of a ventricular premature depolarization when the HIS is refractory cannot advance the atrial activation. B: during atypical atrioventricular nodal reentrant tachycardia, following cessation of ventricular entrainment pacing, the tachycardia resumes with a V-A-V response. The values of post-pacing interval (PPI) minus tachycardia cycle length (TCL) were greater than 115 msec and the ΔHA=40 msec. HRA: high right atrium, HIS: His bundle, CS: coronary sinus, ABL: ablation, RVA: right ventricular apex. |
References
1. Chen YJ, Chen SA, Chiang CE, et al. Dual AV node pathway physiology in patients with Wolff-Parkinson-White syndrome. Int J Cardiol. 1996. 56:275–281.
2. Becker AE, Anderson RH, Durrer D, Wellens HJ. The anatomical substrates of wolff-parkinson-white syndrome. A clinicopathologic correlation in seven patients. Circulation. 1978. 57:870–879.
3. Jackman WM, Wang XZ, Friday KJ, et al. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med. 1991. 324:1605–1611.
4. Calkins H, Langberg J, Sousa J, et al. Radiofrequency catheter ablation of accessory atrioventricular connections in 250 patients. Abbreviated therapeutic approach to Wolff-Parkinson-White syndrome. Circulation. 1992. 85:1337–1346.
5. Lesh MD, Van Hare GF, Schamp DJ, et al. Curative percutaneous catheter ablation using radiofrequency energy for accessory pathways in all locations: results in 100 consecutive patients. J Am Coll Cardiol. 1992. 19:1303–1309.
6. Haissaguerre M, Gaita F, Fischer B, Egloff P, Lemetayer P, Warin JF. Radiofrequency catheter ablation of left lateral accessory pathways via the coronary sinus. Circulation. 1992. 86:1464–1468.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download