Abstract
Arrhythmias can develop in various cardiac diseases, such as ischemic heart disease, cardiomyopathy and congenital heart disease. It can also contribute to the aggravation of heart failure and sudden cardiac death. Redox stress and Ca2+ overload are thought to be the important triggering factors in the generation of arrhythmias in failing myocardium. From recent studies, it appears evident that Ca2+/calmodulin-dependent protein kinase II (CaMKII) plays a central role in the arrhythmogenic processes in heart failure by sensing intracellular Ca2+ and redox stress, affecting individual ion channels and thereby leading to electrical instability in the heart. CaMKII, a multifunctional serine/threonine kinase, is an abundant molecule in the neuron and the heart. It has a specific property as "a memory molecule" such that the binding of calcified calmodulin (Ca2+/CaM) to the regulatory domain on CaMKII initially activates this enzyme. Further, it allows autophosphorylation of T287 or oxidation of M281/282 in the regulatory domain, resulting in sustained activation of CaMKII even after the dissociation of Ca2+/CaM. This review provides the understanding of both the structural and functional properties of CaMKII, the experimental findings of the interactions between CaMKII, redox stress and individual ion channels, and the evidences proving the potential participation of CaMKII and oxidative stress in the diverse arrhythmogenic processes in a diseased heart.
Some pathologic cardiac conditions, including ischemic heart disease, cardiomyopathy and congenital heart disease, are likely associated with arrhythmias, which can lead to sudden cardiac death or may worsen heart failure.
Despite considerable advances in medical treatments for cardiovascular diseases, the development of widely acceptable therapies that can work both for arrhythmia and other associated cardiac diseases remains to be an unsolved issue. The treatment for heart failure may aggravate the associated arrhythmias, or antiarrhythmic drugs, such as ion channel blockers, and are likely to have proarrhythmic side effects in a diseased heart.1)2) Therefore, new treatments targeting both arrhythmia and heart failure are essential, and the understanding of cellular and molecular mechanisms that underlie the pathogenesis of arrhythmias in a diseased heart is thought to be the first step of this development.
This review provides the understanding of pathogenesis of arrhythmias related to Ca2+/calmodulin-dependent protein kinase II (CaMKII) and redox stress, which are proven to be associated with a failing heart, both in human and animal studies.
The impairment of Ca2+ balance and the overproduction of reactive oxygen species (ROS) in diseased cardiac cells are thought be important factors contributing to arrhythmias in a failing heart.3)4) Even though the underlying mechanisms as to how Ca2+ and ROS can produce arrhythmias are still elusive, CaMKII-mediated pathway is a convincing candidate to drive this pathologic process.
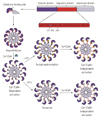
Ca2+/calmodulin-dependent protein kinase II is a multifunctional serine/threonine kinase in which the structural states determine the enzyme activities. This kinase is a multimeric protein, which consists of 12 subunits. Each subunit contains three distinct domains: an association domain which directs the assembly of holoenzyme, a regulatory domain which controls the activation of the enzyme, and a catalytic domain which interacts with the substrates and performs kinase functions (Fig. 1).
In the resting state, CaMKII is bent at the area between the regulatory domain and the catalytic domain, leading to a close association between these two domains. This resting state structure prevents the binding of a substrate to the catalytic domain, resulting in the autoinhibition of kinase activity (Fig. 1).5)
When intracellular calcium concentration rises, calcium-calmodulin binding increases. This calcified calmodulin (Ca2+/CaM) can bind to CaMKII at the regulatory domain and disrupts the association between the regulatory and catalytic domains, leading to a conformational change that exposes a catalytic domain for substrate binding and induces kinase activity (Fig. 1).6)
The activation of CaMKII by Ca2+/CaM binding is transient and readily reversible. Since calcium-calmodulin binding is very sensitive to intracellular Ca2+ concentration, calmodulin becomes immediately decalcified when intracellular Ca2+ concentration returns to a baseline. The decalcification of calmodulin causes a dissociation of calmodulin from CaMKII, resulting in a reassociation of the regulatory and catalytic domains as well as the inactivation of the kinase.
Neuroscience studies investigating learning and memory have found that CaMKII, which is an abundant and major synaptic protein in the neuron, plays a central role in memory storage in brain via long-term potentiation (LTP) process whereby brief-periods of synaptic activity can produce a long-lasting increase in the strength of a synapse.7)
Ca2+/calmodulin-dependent protein kinase II can act as a protein switch; once activated (turned-on) by Ca2+/CaM binding, the enzyme can be autophosphorylated at T287 within the regulatory domain in the presence of sufficient amounts of adenosine triphosphate (ATP) in the cell, an event that produces persistent activation (keeping turned-on state) of CaMKII even after the Ca2+ concentration falls to baseline levels (Fig. 1).8) This is the underlying mechanism of LTP, a key process of memory storage in the brain. For this reason, we call the enzyme "a memory molecule".
In the heart, CaMKII can phosphorylate a diverse array of ion channel proteins, thereby affecting the electrical activities of the myocardium.9) Calcium-dependent facilitation (CDF) of L-type Ca2+ channel is a typical example of interaction between CaMKII and ion channels in cardiac myocytes. CDF is a dynamic positive feedback mechanism in physiologic condition, which augments L-type Ca2+ currents in response to increased intracellular Ca2+ concentrations.10-12) This reaction contributes to cardiac force-frequency relationship; a physiologic phenomenon in which an increased heart rate leads to the augmentation of cardiac contractility. The activation of CaMKII by Ca2+/CaM binding and then the autophosphorylation at T287 is an essential reaction for CDF. Unlike in a neuron, autophosphorylation-mediated CaMKII activation is not persistent in the heart, seeing that CDF lasts only several seconds. This is due in part to the actions of cardiac protein phosphatases, such as protein phosphatase (PP) 1 and PP2A, whereby autophosphorylated CaMKII and phosphorylated ion channels can be easily dephosphorylated in physiologic conditions.
Recently, a new mechanism of CaMKII activation by redox stress was reported, which is likely related to the pathologic conditions in the heart.13) An increased ROS level causes oxidative modification of M281/282 pair within the regulatory domain, blocking the reassociation of the regulatory and catalytic domains and preserving the enzyme activity via a similar but parallel mechanism to T287 autophosphorylation (Fig. 1).
In our previous study, direct perfusion of isolated rat cardiomyocytes with ROS-containing solutions caused large increases in L-type Ca2+ currents via the CaMKII-mediated pathway.14) In contrast to CDF, brief-periods (5 minutes) of ROS exposure produced persistent (>1 hour) rises in L-type Ca2+ currents in rat cardiomyocytes, probably through oxidation-mediated CaMKII activation, suggesting that CaMKII senses redox stress and thereby acts as "a memory molecule" in the heart.15)
In heart failure, neurohormonal systems, including rennin-angiotensin-aldosterone system (RAAS) and β-adrenergic system, are known to be activated to increase the blood pressure in order to maintain tissue perfusion. Chronic elevation of neurohormonal activities appears to cause diverse pathologic processes in the heart, including arrhythmias.
Rennin-angiotensin-aldosterone system is associated with oxidation- and inflammation-processes in the heart.16) Angiotensin II is known to stimulate L-type Ca2+ currents, Na+/Ca2+ exchanger (NCX), and ROS production, assuming that Angiotensin II may affect Ca2+ and ROS homeostases in cardiac cells.17)18) CaMKII appears to act as a key mediator in the interactions of Angiotensin II with intracellular Ca2+ and ROS.
An experimental study revealed that ROS-mediated CaMKII activation via methionine (M281/282) oxidation was a key reaction in Angiotensin II-directed cardiac damages.13) They suggested that oxidative stress is the main factor for Angiotensin II-induced CaMKII activation, even though Angiotensin II can increase both the intracellular Ca2+ and ROS levels.
β-adrenergic receptor agonists increases intracellular Ca2+ concentration via facilitation of L-type Ca2+ currents, ryanodine receptor (RyR) Ca2+ release, and protein kinase A (PKA)-induced Ca2+ influx, thereby leading to CaMKII stimulation.19)
The activation of β-adrenergic receptor can also stimulate CaMKII through Epac (cAMP-dependent exchange protein), which links with CaMKII and β-arrestin at the β-adrenergic receptor.20) Additionally, the stimulation of PKA and phospholamban (PLN) by β-adrenergic receptor agonist seems to directly activate CaMKII.21) In contrast to RAAS, there is no evidence that the β-adrenergic receptor affects methionine oxidation-induced CaMKII stimulation, even though the β-adrenergic receptor is responsible for the cardiac redox state.13)
The expression level of CaMKII is shown to be elevated in cardiac diseases.22) One possible mechanism for this phenomenon is that calcineurin (a PP) is activated in heart failure and stimulates CaMKII expression.23) An experimental study revealed that the overexpression of calcineurin increased CaMKII activity in the heart and led to heart failure and sudden cardiac death in mice, implying that calcineurin plays an important role in the activation of CaMKII as well as in the pathologic processes in a failing heart.24)
L-type Ca2+ channel is the main Ca2+ influx pathway in cardiac myocytes. L-type Ca2+ channel is very closely located to the RyR channel, which is activated by a Ca2+ influx through the L-type Ca2+ channel and then performs the Ca2+ release from sarcoplasmic reticulum (SR), resulting in a large elevation of cytoplasmic Ca2+ concentrations, "Ca2+ transients".
Ca2+/calmodulin-dependent protein kinase II can increase ICa.L and thereby can engender Ca2+ overload in the cardiac cell, which may increase the risk for cardiac diseases, including arrhythmias.25)
Interestingly, the activation of CaMKII in this reaction is mediated by SR Ca2+ release instead of Ca2+ influx through L-type Ca2+ channel, as evidenced by the fact that the prevention of SR Ca2+ release completely blocks CaMKII-mediated ICa.L facilitation.26)
L-type Ca2+ channel consists of α (pore-forming) and β (regulatory) subunits. Even though CaMKII can interact with various sites on α and β subunits, the phosphorylation of T498 on the β subunit is a key reaction for ICa.L facilitation by CaMKII.26)27)
As shown in our previous study, ROS causes ICa.L facilitation in rat ventricular myocytes, which is prevented by potent Ca2+ chelators or CaMKII blockers, implying that this reaction is dependent on the CaMKII pathway.14) Additionally, endothelin-1 and aldosterone, which can activate NADPH oxidase and thereby increase ROS production, also facilitate ICa.L.28)29)
These findings are well consistent with the theory that ROS activates CaMKII via oxidation reaction and that this activated CaMKII increases ICa.L via phosphorylation of the β subunit.
Voltage-gated Na+ currents (INa) play many roles in the initiation and duration of the action potential (AP) in the heart. INa is comprised of a large inward component and a small noninactivating component. The abnormal rise in the noninactivating component of INa is the main cause of long QT syndrome 330) and the potential arrhythmogenic risk factor in a failing heart.31) The rise in INa increases AP duration and intracellular Na+ concentrations, and thereby inhibits the NCX, resulting in intracellular Ca2+ increase. These changes by abnormal INa can produce early and late afterdepolarizations, which are proarrhythmic electrical anomalies.
Voltage-gated Na+ channel can be affected by CaMKII.32) A recent study reported that CaMKII increased the noninactivating component of INa in rabbit ventricular myocytes. These reactions were inhibited by CaMKII blockers.33)
Another study reported that S571 on the α subunit of Na+ channel is a phosphorylation site for CaMKII and that βIV spectrin has an important role in the interaction between CaMKII and the Na+ channel in the heart.34)
Previous studies reported that ROS had phenotypically similar effects on INa to CaMKII in dog, pig, and rabbit heart.35)36) There can be two possible mechanisms to explain these findings. First, as noted above, ROS can activate CaMKII, and then the activated CaMKII interacts with the voltage-gated Na+ channel, leading to INa changes. Second, ROS may directly affect INa in CaMKII-independent manner, seeing that the oxidation of Met residue of the Na+ channel was shown to increase the gating of this channel.37)
Voltage-gated K+ currents (IK) play important roles in membrane repolarization in the heart and have a major influence on the AP shape and duration. Therefore, abnormal IK can give rise to diverse arrhythmias by changing the AP configuration and duration.
Ca2+/calmodulin-dependent protein kinase II has complex effects on IK through transcriptional regulation, gating change via phosphorylation, and control of trafficking to sarcolemmal compartments.38-40) Chronic overexpression of CaMKII in mice increases the slow transient outward K+ currents (Ito,s) and decreases the fast transient outward K+ currents (Ito,f) and inward rectifying K+ currents (Ik1), resulting in the prolongation of AP duration.41)42) In contrast, chronic inhibition of CaMKII was shown to upregulate Ito,f and Ik1, leading to the shortening of AP.41)43)
Sarcoplasmic reticulum Ca2+ uptake and release are the major Ca2+ cycling process in the myocardium and the main determinant of cytoplasmic Ca2+ concentrations. This process is regulated by RyR, PLN and SR Ca-ATPase (SERCA).
Ryanodine receptor is a SR Ca2+ release channel, which is opened by Ca2+ influx via ICa.L. RyR is a proven target of CaMKII.47) CaMKII can phosphorylate S2814 on RyR and thereby induce Ca2+ release from SR, leading to increases in cytoplasmic Ca2+ concentrations and development of delayed afterdepolarizations, which are potentially arrhythmogenic.48) ROS are shown to trigger SR Ca2+ leak and thereby cause arrhythmia in dogs.49) However the involvement of CaMKII in the interaction between ROS and RyR is unclear yet.
Phospholamban is an inhibitor for SERCA, which is a SR Ca2+ uptake channel.50) PLN can be phosphorylated by CaMKII; moreover, the phosphorylation of PLN attenuates the PLN activity, leading to the elevation of SR Ca2+ uptake by SERCA.51) In some animal study, the blocking of PLN by CaMKII-mediated phosphorylation caused sudden cardiac death, even though the underlying mechanisms are unclear yet.52) There is no evidence of a direct interaction between CaMKII and SERCA.
The cardiac beat is directed by pacemaker cells. These cells have a specific property to create electrical impulses by themselves without electrical triggering. This automatic property is inhibited during excitation and refractory periods. Therefore, the most rapid pacing cell, normally sinoatrial (SA) nodal cell, controls the cardiac rhythm, while the other slower automatic cells act just as conduction systems, since they are already excited by the conducted impulse from the SA node before showing automaticity. The defect in SA node, or abnormal ectopic automatic cell with rapid firing rate, can develop arrhythmias.
Ca2+/calmodulin-dependent protein kinase II and redox stress can affect the SA nodal function. ROS has been shown to raise the depolarizing frequency of SA node in animal studies.53)54) The activation of CaMKII also leads to the rise of the depolarizing rate in the SA node. Conversely, blocking of CaMKII decreases ICa.L in the SA node, resulting in a decline of depolarizing frequency.33)55)
A recent animal study showed that Angiotensin II, which is known to be activated in cardiac diseases, induces oxidative modification of CaMKII, increases apoptosis in the atrioventricular node, and reduces the heart rate in various conditions.56) Among cardiac disease patients, oxidized-CaMKII level of the atrium was higher in the SA node dysfunction group than in the intact SA node group. Both the inhibition of NADPH oxidase and the suppression of CaMKII were shown to prevent Angiotensin II-mediated SA node damage, suggesting that Angiotensin II activates CaMKII via NADPH oxidase pathway, thereby leading to SA node dysfunction.56) Interestingly, CaMKII is also involved in the physiological interaction between SA node and β-adrenergic signaling.57)
Taken together, CaMKII and redox stress play critical roles in the SA node dysfunction in a failing heart.
Atrial fibrillation is the major rhythm disturbance in heart failure.58) In the presence of atrial fibrillation, ROS levels in atrial tissues were elevated both in animal and human studies.59)60) The overexpression of CaMKII in the atrium was observed in atrial fibrillation patients.61)
One potential hypothesis to explain atrial fibrillation is that the dysfunction of junctional conduction system may lead to disturbances in electrical impulse spread, likely resulting in atrial fibrillation.62) A recent study showed that ROS interrupted the action of microtubule in the cell and thereby prevented the migration of connexon43 (an important junctional channel) to the junction of the cell, which is essential for the intercellular impulse conduction.63) This finding provides a model showing how the oxidative stress can cause atrial fibrillation.
Another theory for atrial fibrillation is that the dysfunction in RyR may cause abnormal Ca2+ release and give rise to spontaneous Ca2+ sparks, which is proarrhythmic and thus, potentially contributes to atrial fibrillation. As noted above, RyR is a well-known target of both CaMKII and ROS. Therefore, abnormal SR Ca2+ release mediated by CaMKII and ROS can be another possible model for atrial fibrillation in a diseased heart.64)65)
Both congenital and acquired heart diseases, such as cardiac hypertrophy, cardiomyopathy, and ischemic heart disease, can be associated with ventricular arrhythmias. In an animal study, the overexpression of CaMKII was shown to cause ventricular hypertrophy and ventricular tachycardia.66) CaMKII blockers had antiarrhythmic effects in the animal model with cardiomyopathy and ventricular tachycardia caused by the overexpression of PP.24) An experimental animal model of ischemic heart disease with ventricular arrhythmias showed increases in autophosphorylated- and oxidized-CaMKII; further, the blocking of CaMKII reduced arrhythmias in this model.67) These results indicate that CaMKII and redox stress likely mediate, at least in part, the generation of ventricular arrhythmias in cardiac diseases.
The above-reviewed interactions of CaMKII and redox stress with ion channels can be the underlying mechanisms for the genesis of ventricular arrhythmias in a diseased heart.
Ca2+/calmodulin-dependent protein kinase II overexpressing animal with cardiomyopathy and ventricular tachycardia had increased-ICa.L, afterdepolarizations, and AP prolongation, which were reversed by CaMKII blocking.66) These results implied that the interaction between CaMKII and L-type Ca2+ channel plays an important role in the occurrence of ventricular tachycardia in a diseased heart. A heart failure model with ventricular arrhythmias showed an overexpression of CaMKII, hyperphosphorylation of RyR, and rises in SR Ca2+ release, which were prevented by CaMKII inhibition.48)68) Hence, rises in SR Ca2+ release by CaMKII can engender ventricular arrhythmias in cardiac disease. In addition, phosphorylation of the Na+ channel by CaMKII leads to AP prolongation and afterdepolarizations, likely leading to ventricular arrhythmias.33) This finding indicates that the interaction of CaMKII and the Na+ channel is another proposed mechanism for the development of ventricular arrhythmias.
Redox stress can increases noninactivating INa, ICa.L and SR Ca2+ release, and thereby causing AP prolongation and afterdepolarizations in the ventricular cells, predisposing to ventricular arrhythmias.36)69) CaMKII blockers can inhibit these proarrhythmic changes induced by redox stress, suggesting that CaMKII activation by redox stress plays important roles in the genesis of ventricular arrhythmias in cardiac disease.
In addition to the dramatic advances in the understanding of interactions between CaMKII and ion channels and the abundant evidences proving involvements of CaMKII and ROS in the genesis of arrhythmias in a failing heart, the discovery of a new mechanism showing how redox stress activates CaMKII gives us one more piece of the puzzle in order to figure out how arrhythmias can be developed in a diseased heart.
However, much remains to be investigated as to how redox stress and Ca2+ loading can cause CaMKII to target the specific ion channels and whether and how other signaling- and redox regulating-systems interact with CaMKII in the development of arrhythmias.
Future investigation on CaMKII and redox stress in the heart will help us to develop a more targeted therapy for arrhythmias in a failing heart.
Figures and Tables
References
1. Krell MJ, Kline EM, Bates ER, et al. Intermittent, ambulatory dobutamine infusions in patients with severe congestive heart failure. Am Heart J. 1986. 112:787–791.
2. Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991. 324:781–788.
3. Tomaselli GF, Barth AS. Sudden cardio arrest: oxidative stress irritates the heart. Nat Med. 2010. 16:648–649.
4. Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev. 2011. 91:889–915.
5. Rosenberg OS, Deindl S, Sung RJ, Nairn AC, Kuriyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005. 123:849–860.
6. Rellos P, Pike AC, Niesen FH, et al. Structure of the CaMKIIdelta/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. PLoS Biol. 2010. 8:e1000426.
7. Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002. 3:175–190.
8. Lisman JE. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci U S A. 1985. 82:3055–3057.
9. Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol. 2009. 54:180–187.
10. Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca(2+)-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circ Res. 1994. 75:854–861.
11. Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994. 267(3 Pt 2):H982–H993.
12. Xiao RP, Cheng H, Lederer WJ, Suzuki T, Lakatta EG. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc Natl Acad Sci U S A. 1994. 91:9659–9663.
13. Erickson JR, Joiner ML, Guan X, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008. 133:462–474.
14. Song YH, Cho H, Ryu SY, et al. L-type Ca(2+) channel facilitation mediated by H(2)O(2)-induced activation of CaMKII in rat ventricular myocytes. J Mol Cell Cardiol. 2010. 48:773–780.
15. Song YH, Choi E, Park SH, et al. Sustained CaMKII activity mediates transient oxidative stress-induced long-term facilitation of L-type Ca (2+) current in cardiomyocytes. Free Radic Biol Med. 2011. 51:1708–1716.
16. Gradman AH. Evolving understanding of the renin-angiotensin-aldosterone system: pathophysiology and targets for therapeutic intervention. Am Heart J. 2009. 157:6 Suppl. S1–S6.
17. Cingolani HE, Villa-Abrille MC, Cornelli M, et al. The positive inotropic effect of angiotensin II: role of endothelin-1 and reactive oxygen species. Hypertension. 2006. 47:727–734.
18. Talukder MA, Endoh M. Pharmacological differentiation of synergistic contribution of L-type Ca2+ channels and Na+/H+ exchange to the positive inotropic effect of phenylephrine, endothelin-3 and angiotensin II in rabbit ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1997. 355:87–96.
19. Boron WF, Boulpaep EL. Medical Physiology: A Molecular and Cellular Approach. 2005. Philadelphia, PA: Saunders;1319.
20. Mangmool S, Shukla AK, Rockman HA. beta-Arrestin-dependent activation of Ca(2+)/calmodulin kinase II after beta(1)-adrenergic receptor stimulation. J Cell Biol. 2010. 189:573–587.
21. Wang W, Zhu W, Wang S, et al. Sustained beta1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ Res. 2004. 95:798–806.
22. Anderson ME. Calmodulin kinase signaling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol Ther. 2005. 106:39–55.
23. Molkentin JD, Lu JR, Antos CL, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998. 93:215–228.
24. Khoo MS, Li J, Singh MV, et al. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation. 2006. 114:1352–1359.
25. Hashambhoy YL, Winslow RL, Greenstein JL. CaMKII-induced shift in modal gating explains L-type Ca(2+) current facilitation: a modeling study. Biophys J. 2009. 96:1770–1785.
26. Koval OM, Guan X, Wu Y, et al. CaV1.2 beta-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc Natl Acad Sci U S A. 2010. 107:4996–5000.
27. Grueter CE, Abiria SA, Dzhura I, et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006. 23:641–650.
28. Wagner M, Rudakova E, Volk T. Aldosterone-induced changes in the cardiac L-type Ca(2+) current can be prevented by antioxidants in vitro and are absent in rats on low salt diet. Pflugers Arch. 2008. 457:339–349.
29. Zeng Q, Zhou Q, Yao F, O'Rourke ST, Sun C. Endothelin-1 regulates cardiac L-type calcium channels via NAD(P)H oxidase-derived superoxide. J Pharmacol Exp Ther. 2008. 326:732–738.
30. Bennett PB, Yazawa K, Makita N, George AL Jr. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995. 376:683–685.
31. Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail. 2007. 9:219–227.
32. Aiba T, Hesketh GG, Liu T, et al. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010. 85:454–463.
33. Wagner S, Dybkova N, Rasenack EC, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006. 116:3127–3138.
34. Hund TJ, Koval OM, Li J, et al. A β(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010. 120:3508–3519.
35. Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: similarities and differences. Am J Physiol Heart Circ Physiol. 2008. 294:H1597–H1608.
36. Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006. 318:214–222.
37. Kassmann M, Hansel A, Leipold E, et al. Oxidation of multiple methionine residues impairs rapid sodium channel inactivation. Pflugers Arch. 2008. 456:1085–1095.
38. El-Haou S, Balse E, Neyroud N, et al. Kv4 potassium channels form a tripartite complex with the anchoring protein SAP97 and CaMKII in cardiac myocytes. Circ Res. 2009. 104:758–769.
39. Li J, Marionneau C, Koval O, et al. Calmodulin kinase II inhibition enhances ischemic preconditioning by augmenting ATP-sensitive K+ current. Channels (Austin). 2007. 1:387–394.
40. Wagner S, Hacker E, Grandi E, et al. Ca/calmodulin kinase II differentially modulates potassium currents. Circ Arrhythm Electrophysiol. 2009. 2:285–294.
41. House SJ, Singer HA. CaMKII-delta isoform regulation of neointima formation after vascular injury. Arterioscler Thromb Vasc Biol. 2008. 28:441–447.
42. Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002. 364(Pt 3):593–611.
43. Timmins JM, Ozcan L, Seimon TA, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009. 119:2925–2941.
44. Goldhaber JI, Liu E. Excitation-contraction coupling in single guinea-pig ventricular myocytes exposed to hydrogen peroxide. J Physiol. 1994. 477(Pt 1):135–147.
45. Su Z, Limberis J, Martin RL, et al. Functional consequences of methionine oxidation of hERG potassium channels. Biochem Pharmacol. 2007. 74:702–711.
46. Tang XD, Daggett H, Hanner M, et al. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol. 2001. 117:253–274.
47. Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004. 94:e61–e70.
48. Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005. 97:1314–1322.
49. Belevych AE, Terentyev D, Viatchenko-Karpinski S, et al. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009. 84:387–395.
50. Mattiazzi A, Kranias EG. CaMKII regulation of phospholamban and SR Ca2+ load. Heart Rhythm. 2011. 8:784–787.
51. Kranias EG, Gupta RC, Jakab G, Kim HW, Steenaart NA, Rapundalo ST. The role of protein kinases and protein phosphatases in the regulation of cardiac sarcoplasmic reticulum function. Mol Cell Biochem. 1988. 82:37–44.
52. Zhang T, Guo T, Mishra S, et al. Phospholamban ablation rescues sarcoplasmic reticulum Ca(2+) handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice. Circ Res. 2010. 106:354–362.
53. Guo J, Giles WR, Ward CA. Effect of hydrogen peroxide on the membrane currents of sinoatrial node cells from rabbit heart. Am J Physiol Heart Circ Physiol. 2000. 279:H992–H999.
54. Satoh N, Nishimura M, Watanabe Y. Electrophysiologic alterations in the rabbit nodal cells induced by membrane lipid peroxidation. Eur J Pharmacol. 1995. 292:233–240.
55. Rigg L, Mattick PA, Heath BM, Terrar DA. Modulation of the hyperpolarization-activated current (I(f)) by calcium and calmodulin in the guinea-pig sino-atrial node. Cardiovasc Res. 2003. 57:497–504.
56. Swaminathan PD, Purohit A, Soni S, et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011. 121:3277–3288.
57. Wu Y, Gao Z, Chen B, et al. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci U S A. 2009. 106:5972–5977.
58. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991. 22:983–988.
59. Dudley SC Jr, Hoch NE, McCann LA, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005. 112:1266–1273.
60. Kim YM, Guzik TJ, Zhang YH, et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005. 97:629–636.
61. Tessier S, Karczewski P, Krause EG, et al. Regulation of the transient outward K(+) current by Ca(2+)/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res. 1999. 85:810–819.
62. Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res. 1999. 84:776–784.
63. Smyth JW, Hong TT, Gao D, et al. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010. 120:266–279.
64. Chelu MG, Sarma S, Sood S, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009. 119:1940–1951.
65. Lo LW, Chen YC, Chen YJ, Wongcharoen W, Lin CI, Chen SA. Calmodulin kinase II inhibition prevents arrhythmic activity induced by alpha and beta adrenergic agonists in rabbit pulmonary veins. Eur J Pharmacol. 2007. 571:197–208.
66. Wu Y, Temple J, Zhang R, et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002. 106:1288–1293.
67. Zhang R, Khoo MS, Wu Y, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005. 11:409–417.
68. Picht E, DeSantiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency-dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol. 2007. 42:196–205.
69. Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced after depolarizations and calmodulin kinase II signaling. Circ Res. 2009. 104:79–86.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download