Abstract
We report a case about a 27-year-old healthy young male who developed syncope during exercise, which was subsequently identified to be attributable to non-sustained polymorphic ventricular tachycardia (VT). Occurrence of polymorphic VT was neither related to a prolonged QT interval nor a fixed short coupling interval. Standard examinations including echocardiography, coronary angiography, isoproterenol infusion study, and cardiac MRI showed no structural heart disease. On the electrophysiology study, activation mapping revealed that a discrete potential preceded the premature ventricular complex (PVC) triggered polymorphic VT, which was recorded just above the pulmonary valve. After radiofrequency ablation at this area, PVC and polymorphic VT disappeared and did not recur after a 2 month follow up.
Polymorphic ventricular tachycardia (VT) in patients with no heart disease can be attributable to catecholamine sensitive polymorphic VT, short coupled variant of torsade de pointes, or idiopathic ventricular fibrillation (VF). Recently some reports demonstrated that idiopathic VF was initiated by different premature beat morphologies and could be treated with catheter ablation. In this case, we observed that polymorphic VT was not related with any heart disease. The only clue was the frequently isolate premature ventricular complex (PVC), which was preceding polymorphic VT. We assumed that PVC might trigger polymorphic VT. This reports describes that the results of ablation in regards to this becomes triggered in patients exhibiting polymorphic VT.
A 27-year-old male presented with an episode of syncope while playing soccer. During the episode of syncope, a pulse was detected and sphincter function was preserved. He had experienced pre-syncope three times during resting status over the most recent 2 months. He had no history of hypertension and diabetes mellitus. He had no family history of sudden cardiac death or syncope. Also, he did not take any medication.
A physical examination revealed that blood pressure was 130/80 mm Hg and heart rate was 78 beat per minute. Other physical examinations were normal. Baseline electrocardiography (ECG) showed a sinus rhythm with frequent PVC and non-sustained polymporphic VT (Fig. 1). QT interval was 370 ms. PVC morphology, which triggers polymorphic VT is uniform and identical to that of isolated PVC. Initiation of polymorphic VT is not related to the fixed coupling interval. There was no QT interval prolongation, ST elevation on the precordial lead, and abnormal notch at QRS wave. Holter monitoring showed frequent PVC greater than 40% a day and frequent non-sustained polymorphic VT, and there was no diurnal variation in their occurrence (Fig. 2). He felt no specific symptoms during polymorphic VT. A laboratory blood study including serum electrolytes showed no abnormalities. An exercise test using a treadmill showed no changes of PVC and non-sustained polymorphic VT frequency. Isoproterenol infusion did not change in corrected QT interval and PVC frequency. There was no remarkable structural heart disease on the echocardiography except for borderline left ventricular dysfunction (left ventricular ejection fraction=52%). Coronary angiography involving right ventricular angiography was normal and coronary spasm was excluded using the provocation test with ergonovine. A cardiac MRI after catheter ablation also showed no remarkable finding involving the right ventricle.
We thought that isolated PVC triggered polymorphic VT and then ablation of the PVC could abolish the genesis of polymorphic VT. Using activation mapping with an adjustable Lasso catheter (Biosense Webster, Diamond Bar, CA, USA) via the SL1 sheath (St. Jude Medical, Daig Division, Minnetonka, MN, USA), which was positioned at the right ventricular outflow tract (RVOT), a discrete potential was detected at the pulmonary artery just above the pulmonary valve, the free wall side of RVOT (Fig. 3). The local potential preceded the QRS onset by 60 ms and showed relatively high amplitude and reversal of polarity (Fig. 4). Radiofrequency ablation was done just above the pulmonary valve with the temperature setting of 60℃. After radiofrequency delivery for 3.2 seconds, PVC was abolished. There was no PVC or non-sustained VT on the ECG and Holter ECG (Fig. 5). Symptoms of vague chest discomfort improved and there was no PVC free of medication after a 2 month follow up visit.
Polymorphic VTs are usually rapid and readily degenerate to VF. Polymorphic VTs in the setting of a prolonged QT interval is often referred to as "torsade de pointes", and it was related to congenital or acquired long QT syndrome. Therefore, polymorphic VT in the absence of organic heart disease is defined if a patient has no clinical history regarding cardiotoxic drugs and no abnormality in blood chemistry, ECG, exercise test, echocardiography and coronary angiography. Polymorphic VT in patients with no heart disease can be categorized into catecholamine sensitive polymorphic VT, short coupled variant of torsade de pointes, and idiopathic VF.1)2)
In this case, the patient had a normal heart, which was not related to an electrolyte abnormality, or structural heart disease (e.g., coronary artery disease, valvular heart disease, cardiomyopathy, arrhythmogenic right ventricular dysplasia). Polymorphic VT in this patient was not aggravated by exercise and PVC with a fixed short coupling interval.
We assumed that the elimination of the PVC might prevent the development of polymorphic VT because it had the same morphology of PVC, which triggered the polymorphic VT. Haïssaguerre et al.3)4) demonstrated that in most cases, VF was initiated by different premature beat morphologies, which are mapped to various locations in the Purkinje system and treated with catheter ablation. In case of four patients with long QT syndrome, premature beats inducing polymorphic VT or VT originated from the RVOT or Purkinje fiber. After ablation of triggers, there has been no recurrence of VF.5) These literatures demonstrated that catheter ablation of ectopic trigger was useful for eliminating or decreasing the incidence of ventricular arrhythmias including idiopathic VT and long QT syndrome.
However, in our case, activation mapping demonstrated an ectopic potential at the pulmonary artery (just above the pulmonary valve, the free wall side of RVOT) which preceded the QRS onset by 60 ms and showed relatively high amplitude and reversal of polarity. It is unusually earlier than the onset of the QRS wave, which means very slow conduction from the focus of PVC to the neighboring ventricular myocardium. After catheter ablation at this ectopic potential, polymorphic VT disappeared. This finding indicated that the ectopic site at the pulmonary artery is the focus of PVC and polymorphic VT.
Ya et al.6) showed in animal hearts that, during embryogenesis, that the distal part of the outflow tract loses its myocardial phenotype and becomes the proximal part of the ascending aorta and pulmonary trunk. The authors also found that myocardial regression continues until after birth, as revealed by the disappearance of the myocardial cuff surrounding the semilunar sinuses. Hasdemir et al.7) demonstrated that in ninety-five consecutive human hearts obtained at autopsy, ventricular myocardial extension (VME) beyond the ventriculo-arterial junction (VAJ) was found in 21 of 95 (22%) patients studied. This study showed that VME into the pulmonary artery or aorta beyond the VAJ was relatively common. However, the prevalence of these muscle extensions was far more common than the prevalence of VTs originating from the aortic sinuses or the main stem of the pulmonary artery. Therefore, the mere existence of VME into the pulmonary artery does not necessarily give rise to VT.
In our cases, the local potential preceded the QRS onset by 60 ms and showed relatively high amplitude and reversal of polarity. As shown in the Lasso catheter located above the pulmonary valve, besides, any other electrogram was not observed except for that of trigger focus. These findings suggest that the presence of the myocardia sleeve in the anterior free wall side of the pulmonary artery is the source of frequent PVC. The weak connection by incomplete regression of the myocardium between the myocardial sleeve and the RVOT myocardium might cause very slow conduction and variable multiple re-entries that develop non-sustained polymorphic VT, which was not related to structural heart disease, prolonged QT and the fixed coupling interval.
Figures and Tables
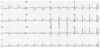
 | Fig. 1Frequent premature ventricular complex and non-sustained polymorphic ventricular tachycardia on baseline electrocardiography. |
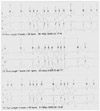 | Fig. 2The sample of the Holter electrocardiography showed frequent non-sustained polymorphic VT. The morphology of triggering PVC is uniform and identical to that of isolated PVC. Development of polymorphic VT is not related to fixed coupling interval of PVC. VT: ventricular tachycardia, PVC: premature ventricular complex. |
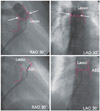 | Fig. 3The position of the ablation catheter and Lasso catheter. The upper panel showed the adjustable Lasso catheter position within the pulmonary artery confirmed by angiography. White arrows indicated the level of the pulmonary valves. The purple line delineated the pulmonary artery and pulmonary valve. The lower panel showed the ablation catheter position during radiofrequency delivery, which was the anterior free wall side just above the pulmonary valve. |
References
1. Viskin S, Belhassen B. Polymorphic ventricular tachyarrhythmias in the absence of organic heart disease: classification, differential diagnosis, and implications for therapy. Prog Cardiovasc Dis. 1998. 41:17–34.
2. Mechleb BK, Haddadin TZ, Iskandar SB, Abboud LN, Fahrig SA. Idiopathic polymorphic ventricular tachycardia with normal QT interval in a structurally normal heart. Pacing Clin Electrophysiol. 2006. 29:791–796.
3. Haïssaguerre M, Shoda M, Jaïs P, et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002. 106:962–967.
4. Haïssaguerre M, Extramiana F, Hocini M, et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003. 108:925–928.
5. Weerasooriya R, Hsu LF, Scavée C, et al. Catheter Ablation of Ventricular Fibrillation in Structurally Normal Hearts Targeting the RVOT and Purkinje Ectopy. Herz. 2003. 28:598–606.
6. Ya J, van den Hoff MJ, de Boer PA, et al. Normal development of the outflow tract in the rat. Circ Res. 1998. 82:464–472.
7. Hasdemir C, Aktas S, Govsa F, et al. Demonstration of ventricular myocardial extensions into the pulmonary artery and aorta beyond the ventriculo-arterial junction. Pacing Clin Electrophysiol. 2007. 30:534–539.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download