Abstract
Background and Objectives
Atrial septal defect (ASD) is the one of most common congenital heart diseases detected in adults. Along with remarkable development of device technology, the first treatment strategy of secundum ASD has been transcatheter closure in feasible cases. However, there are only a few publications regarding the results of transcatheter closure of ASD in elderly patients, especially those over 60 years of age. We report our results of transcatheter closure of ASD in patients over 60 years old.
Subjects and Methods
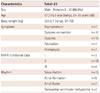
Between May 2006 and December 2011, 31 patients over 60 years old (25 female and 6 male; mean 66.7±5.25 years old, range 61-78 years old) were referred to our center.
Results
A total of 23 patients underwent therapeutic catheterization to close secundum ASD, and the closure was successful in 22 patients (95.7%). All patients who underwent the procedure survived except for one patient who expired because of left ventricular dysfunction. A small residual shunt was observed in two (9%) of 21 patients before discharge but disappeared at follow-up. All patients eventually had complete closure. There were five patients who had coronary problems. One patient underwent percutaneous coronary intervention using a stent at the same time as transcatheter closure of ASD. Atrial arrhythmias were detected in 6 of 23 patients (26.1%) before the procedure. One patient was successfully treated by radiofrequency ablation before the procedure. No patients displayed new onset arrhythmia during the follow-up period. Follow-up echocardiographic evaluation showed a significantly improved right ventricular geometry.
Atrial septal defect (ASD) is the most common congenital heart disease detected in adults, accounting for about 10% of all congenital heart anomalies. Although many patients with ASD are asymptomatic for years, almost all the patients eventually develop symptoms, most often dyspnea on exertion, fatigue, and palpitation. Along with the remarkable developments of device technology, transcatheter closure has been preferred as the initial treatment strategy of secundum ASD in feasible cases.1-3) Moreover, many clinicians have performed transcatheter ASD closure as an alternative to surgery and reported excellent results with low complication rates. However, several factors, such as morphology of ASD, comorbidity, and age, can influence the outcome significantly. In particular, in elderly patients, prolonged left-to-right shunting results in right ventricular (RV) volume overload and subsequent pulmonary hypertension and atrial arrhythmia. We report our results of transcatheter ASD closure with the Amplatzer Septal Occluder (ASO; AGA Medical Corp., Golden Valley, MN, USA) in patients over 60 years old, and discuss the risk factors of the procedure as well as the strategies to prevent complications in this particular subset of patients.
Between May 2006 and December 2011, 31 patients over 60 years of age (25 female and 6 male; mean 66.7±5.25 years old, range 61-78 years old) were referred to our center of Grown-Up Congenital Heart disease. Of these patients, 8 underwent surgery after diagnostic catheterization and 23 underwent transcatheter closure of secundum ASD. The indications for ASD closure were hemodynamically significant secundum ASD <40 mm in diameter with adequate rims (>5 mm in length, except for the anterior superior rim), and RV enlargement on transthoracic echocardiography (TTE) and/or clinically symptomatic patients. ASD assessment was done by TTE and/or transesophageal echocardiography (TEE). Patients who had evidence of partial anomalous pulmonary venous return and/or ASD size >40 mm were excluded from the study. We retrospectively reviewed the medical records of all patients, and the Institutional Review Board approved the study and waived informed consent.
The sizes of defects were measured with TEE or intracardiac echocardiography (ICE) in all patients in the catheterization laboratory. One dose of cephazolin was administered 30 minutes before the procedure and 2 doses were administered 8 hours apart after the procedure. We used ASO in all patients. The procedure was performed under local anesthesia, ICE and fluoroscopic guidance, except for two patients who requested the procedure be done under general anesthesia. A 24 or 34 mm sizing balloon (AGA medical Corp., Golden Valley, MN, USA) was used to measure the diameter of the defect. Baseline hemodynamic assessment was performed in every patient prior to closure except in 1 patient who was evaluated for the hemodynamic state at another hospital and referred to our hospital. We used the stop-flow technique in general to avoid oversizing the defect. Before deployment of the device, we performed the "Minnesota wiggle"(reference) procedure gently to confirm the stability of the device. All patients received aspirin (100 mg) for 6 months and were checked by chest X-ray, electrocardiography and TTE before discharge. We recommended prophylactic antibiotics for infective endocarditis for 6 months. We performed test occlusion of the defect in 3 patients to evaluate the left ventricular compliance in case of high left atrial pressure. One patient's left atrial pressure was 23 mm Hg and the increase of left atrial pressure from baseline was lower than 3 mm Hg. Therefore, we occluded the defect. Another case also involved test occlusion because of high left ventricular end diastolic pressure of 19 mm Hg, which increased to 21 mm Hg on test occlusion. In the 3rd case, test occlusion was performed for left ventricular diastolic dysfunction on TTE. The left ventricular end-diastolic pressure increased from 10 mm Hg to 15 mm Hg after test occlusion and we occluded the defect with heart failure medications after the procedure.
We performed history taking, physical examination, electrocardiography, chest X-ray and TTE at the first post-procedure day, at 1 month, 6 months, 1 year and annually thereafter to assess the symptoms and detect any complications or residual shunt. Residual shunt was classified according to the color jet width described by Boutin et al.4) as trace <1 mm, small >1 mm and <2 mm, moderate >2 mm and <4 mm, large if >4 mm.
The results are presented as mean and range. Differences between pre- and post-closure parameters were compared using Statistical Package for the Social Sciences (SPSS) 13.0 (SPSS Inc., Chicago, IL, USA). Null of no difference were rejected if p were less than 0.05. In patients with multiple ASDs, the largest defect size was used for analysis.
Twenty-two patients had single ASD and 1 patient had two ASDs. The baseline clinical data are presented in Table 1 and the hemodynamic data and related conditions are listed in Table 2.
Percutaneous transcatheter closure was tried in 23 patients and was successful in 22 patients (95.7%). The mean fluoroscopy time was 26.7±13.7 minutes (range 7.6-67 minutes). There was no fenestration case of ASO.
The procedure was successful in all patients except in one who failed due to encroachment of the mitral valve (MV) by the ASO device and was sent to surgery. All patients survived except for one patient who expired the next day of the procedure because of suspicious left ventricular dysfunction that developed after the transcatheter closure of ASD.
A small residual shunt was observed in 2 (9%) of 21 patients on the first follow-up TTE before discharge but disappeared later during the follow-up period. All patients eventually had complete closure.
We used a single ASO in all cases. In one patient with multiple ASD, we occluded the larger one but left the smaller one (2 mm) because of high left atrial pressure (mean 11 mm Hg) and high left ventricular end diastolic pressure (14 mm Hg).
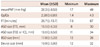
The mean size of ASDs measure by ICE (n=21) or TEE (n=2) was 18.0 mm (range, 11-34 mm), and the mean ASD balloon-stretched diameter (stop-flow technique) (n=18) was 17.8 mm (range, 12-28 mm). The mean of mean pulmonary artery pressure (mean PAP) was 28.3 mm Hg (range, 17-49 mm Hg). The mean pulmonary blood flow to systemic flow ratio as calculated using the Fick's principle was 2.28 : 1 (range, 1.4-4.3 : 1). The mean device size used on all patients (n=22) was 19.9 mm (range, 12-32 mm).
Pulmonary arterial hypertension (mean PAP ≥25 mm Hg) was observed in 15 patients (65.2%). Of these, 3 patients had significant pulmonary hypertension (mean PAP ≥40 mm Hg). All patients with pulmonary hypertension underwent transcatheter closure of ASD with successful results except for 1 patient who underwent surgical closure because the device encroached on the MV. There were no significant complications except pain, bruising or hematoma at the site of venous puncture. However, a patient who showed successful results in immediate post-procedural assessment developed cardiac arrest after one day of the procedure and expired despite repeated resuscitation efforts.
There were 5 patients with coronary problems, thus they were referred to adult cardiologist. 4 patients underwent medical therapy and 1 patient underwent percutaneous coronary artery intervention using a stent because of 90% stenosis of the right coronary artery at the same time of transcatheter closure of ASD.
Atrial arrhythmias were detected in 6 of 23 patients (26.1%, 5 atrial fibrillation, 1 atrial flutter) before the procedure. 1 of 6 patients was successfully treated by radiofrequency ablation before transcatheter closure of ASD and 5 patients were managed with antiarrhythmic medications. 2 patients were reported to have persistent atrial fibrillation in the follow-up period. No patients had new onset arrhythmia during the follow-up period.
We followed-up on 21 of 23 patients. 1 patient expired the next day of the procedure and 1 patient underwent operation. The mean follow-up interval was 21.6±18.5 months (range, 2.4-59 months). Patients were reassessed for New York Heart Association (NYHA) functional class and we discovered that 18 (85.7%) of 21 patients showed improvement. 3 patients showed no change but they showed NYHA functional class I before the procedure (Table 3).
We evaluated geometric changes in the RV by measuring tricuspid valve (TV) diameter and TV/MV diameter ratio (TV/MV ratio) and we found a statistically significant improvement of RV geometry after closure of the defect. The mean TV/MV ratio was decreased from 1.31±0.2 before transcatheter closure of ASD to 1.10±0.17 after transcatheter closure. The mean TV diameter was decreased from 38.3±3.63 mm before transcatheter closure to 34.4±4.83 mm after transcatheter closure (Table 4). These changes in the RV geometry did not include patients who underwent operation or expired.
Percutaneous transcatheter closure of secundum ASD with the ASO in pediatric and adult patients has a high success rate and excellent results which have been documented in many studies.2)3) Moreover, lower rates of mortality and perioprative complications are observed in patients less than 40 years of age.5) However, there are only a few available data on elderly patients of age older than 60 years. Surgical closure of ASD in patients over 60 years old can result in significant mortality and morbidity.6) A study showed that patients who underwent surgical therapy had improved functional class and better survival rate than those treated with conservative therapy.7-9)
One of the most difficult problems for treatment of ASD in elderly patients is comorbid disease. More than one third of patients have systemic hypertension and other systemic diseases such as diabetes mellitus or cerebral infarction. Cardiac comorbidities such as pulmonary hypertension, ventricular dysfunction, atrial arrhythmia and ischemic heart disease are known to make the problem more complicated.10-14)
This retrospective study was performed to evaluate the immediate and midterm results of transcatheter closure of ASD with ASO in patients over 60 years of age and to analyze symptomatic improvement, RV dimension, associated comorbidities and complications. The procedure was successful in all patients except in one who failed due to encroachment of the MV by the ASO device. All patients survived except for one patient who expired the day after the procedure due to a suspicious left ventricular dysfunction that developed after the transcatheter closure of ASD. As with many authors, we recommend test occlusion of the defect in patients with baseline mean left atrial pressure ≥15 mm Hg or left ventricular end-diastolic pressure ≥18-20 mm Hg for 15-20 minutes, and then recheck the mean left atrial pressure or left ventricular end-diastolic pressure. If the pressure is increased by more than 5 mm Hg, closure of ASD should be performed with ASO with a small fenestration or treatment should begin with heart failure medications. Unfortunately, the patient in the study that has expired had mildly high left atrial pressure (11-12 mm Hg) and left ventricular end-diastolic pressure (17 mm Hg) and thus we did not perform test occlusion. The accurate cause of death of this patient is not known. She had mild pulmonary hypertension with a severely enlarged right ventricle (TV diameter=45.7 mm) but no coronary artery problem and no arrhythmia. Therefore, we cannot exclude the possibility of masked restriction of the left ventricle. In such cases, prolonged left to right shunting may result in RV volume overload and mask the restriction of the left ventricle. Therefore, we recommend careful hemodynamic assessment in elderly patients with moderate to large ASDs for potential left ventricular dysfunction as well as temporary balloon test occlusion of ASD before transcatheter closure especially in cases of more than 10 mm Hg of left atrial pressure, and we also recommend the commencement of anticongestive medications in high risk patients.9-11)15-19)
In this study, we used ICE in 21 (91.3%) of 23 patients. The femoral vein was punctured in 2 separate sites, 1 for the device delivery system and 1 for the ICE catheter. We usually use ICE as an imaging guidance tool because there is no need for endotracheal intubation and general anesthesia. There were no ICE-associated complications except pain, bruising or hematoma at the site of venous puncture, and we obtained images of superior quality. Therefore, if available and familiarized, ICE provides better images during transcatheter closure of ASD.20)21)
In this study, 6 (26%) of 23 patients had documented atrial fibrillation or flutter prior to ASD closure. The occurrence of arrhythmia in this group may be due to prolonged right atrial dilatation caused by prolonged left-to-right shunting. Chessa et al.22) reported arrhythmic problems after transcatheter closure of ASD due to stretching of the interatrial septum by the central waist of the device. In this study, however, we had no new onset of atrial fibrillation or flutter.
Some reports have documented that substantial reverse remodeling of the heart after closure improves the symptoms and functional status.11)12)16) From this study, we found a statistically significant decrease in TV dimension and TV/MV ratio which suggest decrease in RV volume after transcatheter closure of ASD as well as improved functional status despite the old age of the patients.
This study has some limitations such as the retrospective nature of the study, small sample size and relatively short follow-up period. A study with a larger sample may produce a larger number of adverse events.
In conclusion, even in patients over 60 years of age, transcatheter closure of ASD is a safe and an effective treatment method if the procedure is performed under a thorough evaluation including comorbidities, risk factors and possibility of left ventricular dysfunction. Thus, we recommend the temporary balloon test occlusion of ASD and careful observation in the change of left atrial pressure or left ventricular end-diastolic pressure. Also, physician must follow their patients for serious complications such as arrhythmia, pulmonary hypertension, and right and left ventricular failure. We can also expect right heart remodeling in patient over 60 years old.
Figures and Tables
References
1. Podnar T, Martanovic P, Gavora P, Masura J. Morphological variations of secundum-type atrial septal defects: feasibility for percutaneous closure using Amplatzer septal occluders. Catheter Cardiovasc Interv. 2001. 53:386–391.
2. Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K. Amplatzer Investigators. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002. 39:1836–1844.
3. Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005. 45:505–507.
4. Boutin C, Musewe NN, Smallhorn JF, Dyck JD, Kobayashi T, Benson LN. Echocardiographic follow-up of atrial septal defect after catheter closure by double-umbrella device. Circulation. 1993. 88:621–627.
5. Murphy JG, Gersh BJ, McGoon MD, et al. Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med. 1990. 323:1645–1650.
6. Harjula A, Kupari M, Kyösola K, et al. Early and late results of surgery for atrial septal defect in patients aged over 60 years. J Cardiovasc Surg (Torino). 1988. 29:134–139.
7. Konstantinides S, Geibel A, Olschewski M, et al. A comparison of surgical and medical therapy for atrial septal defect in adults. N Engl J Med. 1995. 333:469–473.
8. Miyaji K, Furuse A, Tanaka O, Kubota H, Ono M, Kawauchi M. Surgical repair for atrial septal defect in patients over 70 years of age. Jpn Heart J. 1997. 38:677–684.
9. Attie F, Rosas M, Granados N, Zabal C, Buendía A, Calderón J. Surgical treatment for secundum atrial septal defects in patients >40 years old. A randomized clinical trial. J Am Coll Cardiol. 2001. 38:2035–2042.
10. Schubert S, Peters B, Abdul-Khaliq H, Nagdyman N, Lange PE, Ewert P. Left ventricular conditioning in the elderly patient to prevent congestive heart failure after transcatheter closure of atrial septal defect. Catheter Cardiovasc Interv. 2005. 64:333–337.
11. Swan L, Varma C, Yip J, et al. Transcatheter device closure of atrial septal defects in the elderly: technical considerations and short-term outcomes. Int J Cardiol. 2006. 107:207–210.
12. Spies C, Hijazi ZM. Transcatheter closure of secundum atrial septal defects in the elderly. Korean Circ J. 2009. 39:47–51.
13. Giardini A, Donti A, Sciarra F, Bronzetti G, Mariucci E, Picchio FM. Long-term incidence of atrial fibrillation and flutter after transcatheter atrial septal defect closure in adults. Int J Cardiol. 2009. 134:47–51.
14. Taniguchi M, Akagi T, Ohtsuki S, et al. Transcatheter closure of atrial septal defect in elderly patients with permanent atrial fibrillation. Catheter Cardiovasc Interv. 2009. 73:682–686.
15. Ewert P, Berger F, Nagdyman N, et al. Masked left ventricular restriction in elderly patients with atrial septal defects: a contraindication for closure? Catheter Cardiovasc Interv. 2001. 52:177–180.
16. Elshershari H, Cao QL, Hijazi ZM. Transcatheter device closure of atrial septal defects in patients older than 60 years of age: immediate and follow-up results. J Invasive Cardiol. 2008. 20:173–176.
17. Kim NK, Park SJ, Choi JY. Transcatheter closure of atrial septal defect: does age matter? Korean Circ J. 2011. 41:633–638.
18. Holzer R, Hijazi ZM. Interventional approach to congenital heart disease. Curr Opin Cardiol. 2004. 19:84–90.
19. Beyer J. Atrial septal defect: acute left heart failure after surgical closure. Ann Thorac Surg. 1978. 25:36–43.
20. Koenig P, Cao QL, Heitschmidt M, Waight DJ, Hijazi ZM. Role of intracardiac echocardiographic guidance in transcatheter closure of atrial septal defects and patent foramen ovale using the Amplatzer device. J Interv Cardiol. 2003. 16:51–62.
21. Zanchetta M, Onorato E, Rigatelli G, et al. Intracardiac echocardiography-guided transcatheter closure of secundum atrial septal defect: a new efficient device selection method. J Am Coll Cardiol. 2003. 42:1677–1682.
22. Chessa M, Carminati M, Butera G, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol. 2002. 39:1061–1065.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download