Abstract
A 73-year-old man with a history of hypertension and ascending aortic dissection was hospitalized for aggravated abdominal pain and general ache for 3 months. Follow-up CT showed aggravated abdominal aortic hematoma with aneurysm, atherosclerotic periaortitis and bilateral hydronephrosis. An initial laboratory finding showed elevated levels of inflammatory markers and renal dysfunction. Positron emission tomography-CT showed an increased standardized uptake values level in the aortic arch, descending thoracic aorta, major branch, abdominal aorta, and common iliac artery. For bilateral hydronephrosis, a double J catheter insertion was performed. Tissue specimens obtained from previous surgery on the aorta indicated the infiltration of lympho-plasma cells without granuloma formation in the aortic wall. After a combined therapy of high dose steroid therapy with azathioprine, the patient's initial complaints of abdominal pain, weakness and azotemia improved. This case was diagnosed as chronic periaortitis based on aortic inflammation at biopsy, which was complicated with retroperitoneal fibrosis and ureteric obstruction.
The term chronic periaortitis refers to a spectrum of idiopathic diseases characterized by a fibroinflammatory reaction that extends from the adventitia of the abdominal aorta and the common iliac arteries into the retroperitoneum. This often leads to the encasement of the adjacent structure, e.g. ureters or inferior vena cava. Idiopathic retroperitoneal fibrosis, inflammatory abdominal aortic aneurysms and perianeurysmal retroperitoneal fibrosis are its various clinical presentations.1)2) It is most often diagnosed by a periaortic enhancement based on imaging studies, owing to the difficulty of using an aortic wall biopsy. We present a patient who was confirmed with aortic inflammation based on biopsy specimen.
The patient, a 73-year-old man, was admitted with abdominal pain and general ache for 3 months. Eight years ago, a coronary angiogram at another medical center, because of chest pain, showed normal coronary arteries. Three years ago, he started to complain of an intermittent dull pain from the upper abdomen to the chest. A year ago, he visited another medical center with acute chest pain where he was diagnosed as acute aortic dissection, Debakey type I, at that time. He then underwent ascending aorta replacement using a graft. Nine months later, his abdominal pain with general ache worsened. A follow-up chest CT showed an aggravated descending aortic hematoma and bilateral hydronephrosis.
On admission day, his vital signs were stable; blood pressure was 110/68 mm Hg, heart rate 65 beats per minute, body temperature 36.5℃. Physical examination revealed no abnormal findings. The initial laboratory findings showed an elevated level of inflammatory markers; erythrocyte sedimentation rate (ESR) 100 mm/hr (normal range 0-10 mm/h), C-reactive protein (CRP) 4.19 mg/dL (normal range <0.5 mg/dL) and Ferritin 837.5 ng/mL (normal range 30-400 ng/mL). The serum creatinine was 1.82 mg/dL but the urinalysis did not showed overt proteinuria, probably caused by post-renal obstruction. The immunoglobulin G (IgG) level was borderline high, 1627 mg/dL (normal range: 700-1600 mg/dL) and the IgG subtype IV accounted for approximately 4.4%, 0.73 g/L (normal range: 0.06-1.21 g/L). Autoantibodies, such as antinuclear antibody, antineutrophil cytoplasmic autoantibody and rheumatoid factor were negative. Complement three (C3) and four (C4) levels were also between normal range, 138.7/38.6 mg/dL (normal range 90-180 mg/dL, 10-40 mg/dL). The 2D-echocardiography showed normal systolic function and normal sized left ventricle without regional abnormality. The follow-up CT scan showed aggravated abdominal aortic intramural hematoma but also bilateral ureteral obstruction related to retroperitoneal fibrosis. For bilateral hydronephrosis due to ureteral obstruction, we performed a double J catheter insertion at both ureters (Fig. 1). The serum creatinine level had not risen since, but it was also not normalized and remained around 1.8 mg/dL. After consultation with the physician who conducted the chest surgery, we continued medical therapy for chronic abdominal aortic intramural hematoma.
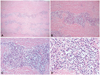
Because of the chronic recurrent disease course and CT finding, we considered the diagnosis of chronic periaortitis with retroperitoneal fibrosis. Chronic periaortitis may present as dull, poorly localized pain, nonspecific systemic illness, raised inflammatory markers and renal impairment due to ureteric obstruction.3-5) Positron emission tomography (PET)-CT showed an increased standardized uptake values level in not only the aortic arch, descending thoracic aorta and major branch (maximum 4.3), but also the abdominal aorta (maximum 5.2) and common iliac artery (maximum 5.3) (Fig. 2). On review of the biopsy specimen achieved from previous surgery on the ascending aorta, the hematoxylin-eosin stain showed a consistent infiltration of lympho-plasma cells without granuloma formation in the media of the aortic wall (Fig. 3). In immunochemistry staining, positive findings were revealed at CD20, CD3, Lambda and Kappa stain.
With the diagnosis of chronic aortitis with retroperitoneal fibrosis complicated to bilateral hydronephrosis, we consulted rheumatology, and high-dose steroid therapy (1 mg/kg prednisolone) and azathioprine (50 mg t.i.d) medication were started. Several days later, his complaints including weakness and abdominal pain improved and laboratory findings demonstrated improving results (ESR 39 mm/hr, CRP 0.09 mg/dL, serum creatinine 1.37 mg/dL). He was discharged and re-visited our institution 2 weeks later for follow up. He complained of weakness and follow up laboratory findings showed extensive elevation of inflammatory markers; ESR 107 mm/hr, CRP 39.28 mg/hr. On that very day, he died a sudden death.
Chronic periaortitis was originally considered a localized inflammatory response to severe aortic atherosclerosis. However, subsequent studies have suggested that chronic periaortitis is a systemic immune-mediated or inflammatory process.6) This is a rare disease with protean manifestations but, if correctly diagnosed, can be successfully managed. Early diagnosis may be important in reducing the mortality.3)4) Clinical features of chronic periaortitis is usually related to the mechanical effects of the retroperitoneal fibrosis on the adjacent structures, such as back or abdominal pain, ureteral colic-like pain, deep vein thrombosis, claudication, and testicular pain. Dull diffuse back or abdominal pain is the commonest symptom (80%). However it also presents with constitutional symptoms, such as fatigue, weight loss and anorexia, probably reflecting the systemic inflammatory nature of the disease.
Laboratory investigations are useful but not diagnostic.5)7)8) The pathologic changes in chronic periaortitis involve both the aortic wall and the surrounding soft tissues. The inflammatory infiltrate mainly consists of mononuclear cells, such as lymphocytes, plasma cells and macrophages and scattered eosinophils, which predominate at the media-adventitia junction and extend throughout the adventitia.6)8) Immunoglobulin G4-related lymphoplasmacytic aortitis is characterized by the enhanced tissue migration of IgG4-positive lymphocytes accompanied by an increase in serum IgG4 levels although this case did not fit that description.9)10) Diagnosis should start by excluding possible primary causes of retroperitoneal fibrosis and is made by means of imaging studies. CT and MRI are the choice modalities for not only the diagnosis but also follow-up. Iino et al.11) have reported a sensitivity of 83.3%, a specificity of 99.7% and an accuracy of 93.7% for the diagnosis of in flammatory abdominal aortic aneurysms using CT.3) PET with 18F-fluorodeoxyglucose (FDG) is emerging as a useful functional imaging modality for assessing disease activity. In the early stages of the disease, there is a strikingly higher periaortic uptake of FDG, which disappears after successful medical therapy.12) This is a rare case which was confirmed as tissue diagnosis, not only an imaging diagnosis with CT finding.
There are no clear guidelines concerning medical treatment. Steroid treatment represents the mainstay of therapy but its role is remains controversial. Recent retrospective analysis has found that the combination of steroids and azathioprine as effective as and safer than steroids plus cyclophosphamide. Steroid, alone or in combination with surgery, is usually effective, but prospective randomized trials are required to establish the best treatment approach.3)4)13) Suspicion of chronic periaortitis is mostly important to treatment of these patients and careful follow-up is essential because of its chronic-relapsing clinical course.
Figures and Tables
Fig. 1
Three retrograde pyelographies showing left ureter obstruction (A), right ureter obstruction (B) and post double J catheter insertion state (C).
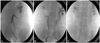
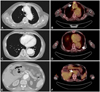
Fig. 2
A computed tomography and a PET CT showing aortic intramural hematoma and FDG uptake at level of thoracic aorta (A and B), descending aorta (C and D) and abdominal aorta (E and F) (the white arrow indicates increased SUV in PET scan image). PET: Positron emission tomography, FDG: Fluorodeoxyglucose, SUV: standardized uptake values.
References
1. Mitchinson MJ. Chronic periaortitis and periarteritis. Histopathology. 1984. 8:589–600.
2. Parums DV. The spectrum of chronic periaortitis. Histopathology. 1990. 16:423–431.
3. Vaglio A, Buzio C. Chronic periaortitis: a spectrum of diseases. Curr Opin Rheumatol. 2005. 17:34–40.
4. Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford). 2004. 43:1441–1446.
5. Vaglio A, Corradi D, Manenti L, Ferretti S, Garini G, Buzio C. Evidence of autoimmunity in chronic periaortitis: a prospective study. Am J Med. 2003. 114:454–462.
6. Vaglio A, Greco P, Corradi D, et al. Autoimmune aspects of chronic periaortitis. Autoimmun Rev. 2006. 5:458–464.
7. Van Bommel EF, Jansen I, Hendriksz TR, Aarnoudse AL. Idiopathic retroperitoneal fibrosis: prospective evaluation of incidence and clinicoradiologic presentation. Medicine (Baltimore). 2009. 88:193–201.
8. Vaglio A, Pipitone N, Salvarani C. Chronic periaortitis: a large-vessel vasculitis? Curr Opin Rheumatol. 2011. 23:1–6.
9. Stone JH, Khosroshahi A, Hilgenberg A, Spooner A, Isselbacher EM, Stone JR. IgG4-related systemic disease and lymphoplasmacytic aortitis. Arthritis Rheum. 2009. 60:3139–3145.
10. Sakamoto A, Nagai R, Saito K, et al. Idiopathic retroperitoneal fibrosis, inflammatory aortic aneurysm, and inflammatory pericarditis: retrospective analysis of 11 case histories. J Cardiol. 2012. 59:139–146.
11. Iino M, Kuribayashi S, Imakita S, et al. Sensitivity and specificity of CT in the diagnosis of inflammatory abdominal aortic aneurysms. J Comput Assist Tomogr. 2002. 26:1006–1012.
12. Blockmans D, Van Moer E, Dehem J, Feys C, Mortelmans L. Positron emission tomography can reveal abdominal periaortitis. Clin Nucl Med. 2002. 27:211–212.
13. Kardar AH, Kattan S, Lindstedt E, Hanash K. Steroid therapy for idiopathic retroperitoneal fibrosis: dose and duration. J Urol. 2002. 168:550–555.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download