Abstract
A paradoxical embolism is defined as a systemic arterial embolism requiring the passage of a venous thrombus into the arterial circulatory system through a right-to-left shunt, and is commonly related to patent foramen ovale (PFO). However, coexisting pulmonary embolisms, deep vein thromboses (DVT), and multipe systemic arterial embolisms, associated with PFO, are rare. Here, we report a patient who had a cryptogenic ischemic stroke, associated with PFO, which is complicated with a massive pulmonary thromboembolism, DVT, and renal infarctions, and subsequently, the patient was treated using a thrombolytic therapy.
A paradoxical embolism (PDE) is defined as a systemic arterial embolism that enters from the venous thrombi into the arterial circulatory system through a right-to-left shunt. The most common right-to-left shunt, associated with PDEs, is patent foramen ovale (PFO), which has been reported as an important cause of cryptogenic strokes.1-3) In addition to the isolated cryptogenic strokes, related to PFOs, the coexistence of pulmonary thromboembolisms (PTE) and deep vein thromboses (DVT) have also been reported,4-6) as rare cases of arterial systemic embolic involvement through PFO.7-9) Here, we report a rare case of cryptogenic stroke, caused by a PDE through PFO, which is complicated with massive PTE, DVT, and renal infarctions.
A 52-year-old woman was transferred to our emergency room (ER) with a 10-hour history of left-side weakness, and a sudden onset of slurred speech, with a clear mental state. Her previous medical, medication, and familial histories were unremarkable, and she was a non-smoker. Upon arrival in the ER, she was hemodynamically stable and her electrocardiogram showed a normal sinus rhythm. However, a magnetic resonance diffusion image of her brain revealed an infarction of the right middle cerebral artery territory (Fig. 1A), and a magnetic resonance angiogram of the cerebral and carotid arteries showed a total obstruction of the genu branch of the right middle cerebral artery (Fig. 1B). She was immediately admitted to the neurologic intensive care unit, with a diagnosis of acute cerebral infarction. Further studies were then ordered, since the etiology of the cerebral infarction was unclear. To evaluate the intracardiac source of the embolism, a transthoracic echocardiogram (TTE) and transesophageal echocardiogram (TEE) were performed after 1 week. The TTE revealed no evidence of embolism; however, the TEE revealed separation of the septum primum from the septum secundum that constitutes a PFO, up to 6 mm, according to the pressure variance (Fig. 2A), and an agitated saline injection test was used to identify the right-to-left microbubble shunting, during a Valsalva maneuver used to confirm the PFO presence (Fig. 2B). Based on these tests, we subsequently recommended an anticoagulation therapy based on warfarinization.
The day after these initial tests, the patient complained of severe dyspnea and chest tightness, while walking. She then displayed severe hypoxemia and hypotension, and was immediately intubated and initiated the ventilator care. A computed tomography (CT) image of her chest was taken after starting the ventilator care, which showed massive thromboembolisms in the right pulmonary artery and left pulmonary segmental artery (Fig. 3A), indicating an acute PTE. Because she was in a hemodynamically compromised state, we considered the use of thrombolytic therapy, in spite of the general contraindication of a recent cerebral infarction. After the discussion with a neurologist, we decided to treat her with a half dosage of recombinant tissue plasminogen activator {rtPA, Actylase® (Boehringer Ingelheim, Ingelheim, Germany); 45 mg total} in order to treat the massive PTE. Within 2-3 hours after an intravenous infusion of the thrombolytic agent, hypoxemia and hypotension symptoms returned to a normal state. After stabilization of her vital signs, we checked for the presence of DVT; the CT venogram of her lower extremities showed thrombi on her left iliac vein and both peroneal veins (Fig. 4A and B). On the arterial phase of this CT, previous thrombi in the right main pulmonary and left segmental arteries were resolved (Fig. 3B). In addition, there were newly developed multiple wedge-shaped hypoperfusion lesions in both kidneys, compatible with an acute renal infarction (Fig. 4C). A work-up of vasculopathies, hypercoagulable states, and hematologic disorders proved unremarkable. We, subsequently, started an oral anticoagulation therapy with warfarin potassium. As a result of this treatment plan, the patient was discharged 20 days after admission, and has since been visiting the out-patient department on a regular basis without recurrence of these ischemic events.
Because there is considerable evidence suggesting that PFO is highly associated with cryptogenic strokes, PFO detection during the evaluation of a patient having a cryptogenic stroke is not surprising. But, when the PFO is detected in a patient having a cryptogenic stroke, the presence of DVT and PTE always have to be a matter of concern. In our case, DVT was detected after the event of a massive PTE, without previous definitive symptoms or signs of DVT. And there was no evidence of coagulopathy, which could cause serious embolic events. As such, we are not able to explain precisely whether these DVT and subsequent PTE were present before the stroke, or produced during her admission and immobilization. However, searching for the coexistence of PTE or DVT and their prophylaxis with anticoagulation could be a more important process. In literature, Clergeau et al.4) demonstrated that PFO is an independent predictor of the silent brain ischemia in patients with PTE, and concluded that patients having cryptogenic stroke and PFO may require a systematic search for silent thromboembolisms. Tanislav et al.10) reported that the silent PTEs frequently occur in patients with cryptogenic strokes and PFOs. Also, the Paradoxical Embolism from Large Veins in Ischemic Stroke study addressed direct evidence of the increased frequency of DVT, among patients with cryptogenic strokes and PFOs.6)
This patient in our case had a recent stroke and experienced a hemodynamically compromised state, due to a massive PTE. In this situation, we had difficulty in making a therapeutic decision between the beneficial effects of thrombolysis on the hemodynamic instability of the massive PTE, and the potentially catastrophic effect of a cerebral hemorrhage. As such, after the discussion with a neurologist, we administrated a half dose of rtPA, and this modified thrombolytic therapy enabled the patient to make a good recovery from hemodynamic instability. In literature, there are number of case reports presenting similar situations.5)11) However, our case was notably different from these cases, both in terms of the timing of the stroke and the treatment modality. Therefore, other therapeutic options, including surgical or catheter embolectomy, must be considered when there are contraindications for systemic thrombolysis, like in our case.12) However, because there was a lack of experience of invasive embolectomy in our center, and these kinds of modalities are traditionally chosen in case of failure of systemic thrombolysis, we chose a systemic thrombolytic therapy. Also, there is no strong evidence to support a particular therapeutic option under the specific conditions of a stroke on a PFO, associated with PTE. Thus, every treatment modality ranging from a more conservative approach with anticoagulation alone to a more aggressive approach with invasive thrombectomy should be considered on a case-by-case basis.
In this case, there was an acute renal infarction, detected on the arterial phase of the CT venogram, suspected to be a result of a PDE; this renal infarction was not detected on the first CT angiogram when the pulmonary embolism was diagnosed. The mechanism of the renal infarction in our case was considered that transiently increased right arterial hypertension, due to acute pulmonary embolism induced continuous opening of PFO and permitted a passage of the thrombi in deep vein into the arterial system.11) It should also be noted that though there have been several case reports of systemic PDEs with PTE associated with PFO,7)8)13) cases of non-cerebral, systemic PDEs, associated with PFO, are relatively lower than for cerebral PDEs. One report showed that only 2.9% of patients with PFO presented with a presumptive diagnosis of systemic embolism,14) though when patients with major pulmonary embolism have PFO, the incidence of peripheral artery embolisms, can significantly be increased (up to 15%).15)
We are following up this patient with anticoagulation using warfarin, and this patient has not shown recurrent cerebral ischemia or thrombotic events. However, the closure of PFO is considered to eliminate the risk of PDE, and to no longer require the use of warfarin. Also, many studies have shown the favorable outcomes of percutaneous device closure of PFO.16)17) So, we plan to recommend a percutaneous PFO closure to this patient, after confirming the nonexistence of DVT for several months.
In conclusion, this report describes a rare case of cryptogenic stroke, associated with PFO and coexisting PTE, DVT, and renal infarctions. When PFO is suspected as a cause of the cryptogenic stroke, it is considered here that seeking the presence of PTE, DVT, and other systemic arterial embolisms, using an imaging modality is useful for the prediction and prevention of adverse outcomes.
Figures and Tables
Fig. 1
Brain MRI and MRA. A: brain MRI reveals a high signal intensity lesion in the right middle cerebral artery in the diffusion image (arrow). B: MRA shows total occlusion of genu of the right middle cerebral artery (arrow). MRA: magnetic resonance angiogram.
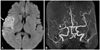
Fig. 2
TEE and TEE with agitated saline test. A: TEE shows a separation of the septum primum from septum secundum that constitutes a PFO (arrow). B: agitated saline injection test identified a right-to-left microbubble shunting during a Valsalva maneuver (arrow). TEE: transesophageal echocardiogram, PFO: patent foramen ovale.
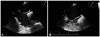
Fig. 3
Chest CT angiogram. A: chest CT shows pulmonary embolism of the right inferior pulmonary artery and left pulmonary segmental artery (arrows). B: 9 hours after thrombolytic therapy, the follow-up CT angiogram shows resolved state of pulmonary embolism (arrows). CT: computed tomography.
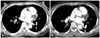
Fig. 4
CT angiogram after thrombolytic therapy. A and B: CT venogram of the pelvis and lower extremities show thrombi in the left common iliac vein (A, arrow) and multiple thrombi in both peroneal veins (B, arrows). C: abdomen CT reveals newly developed multiple wedge-shaped hypoperfusion lesions in both kidneys, compatible with renal infarctions (arrows). CT: computed tomography.

References
1. Webster MW, Chancellor AM, Smith HJ, et al. Patent foramen ovale in young stroke patients. Lancet. 1988. 2:11–12.
2. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988. 318:1148–1152.
3. Oh BH, Park SW, Choi YJ, et al. Prevalence of the patent foramen ovale in young patients with ischemic cerebrovascular disease: transesophageal contrast echocardiographic study. Korean Circ J. 1993. 23:217–222.
4. Clergeau MR, Hamon M, Morello R, Saloux E, Viader F, Hamon M. Silent cerebral infarcts in patients with pulmonary embolism and a patent foramen ovale: a prospective diffusion-weighted MRI study. Stroke. 2009. 40:3758–3762.
5. Pelletier M, Bugeaud R, Ibrahim R, Morency G, Kouz S. Successful thrombolysis of a stroke with a pulmonary embolism in a young woman. J Emerg Med. 2010. 39:443–448.
6. Cramer SC, Rordorf G, Maki JH, et al. Increased pelvic vein thrombi in cryptogenic stroke: results of the Paradoxical Emboli from Large Veins in Ischemic Stroke (PELVIS) Study. Stroke. 2004. 35:46–50.
7. Lim DS, Jeong ES, Jung JS, et al. A case of paradoxical renal embolism through patent foramen ovale. Korean J Nephrol. 2011. 30:667–670.
8. Caretta G, Robba D, Bonadei I, et al. Multiorgan paradoxical embolism consequent to acute pulmonary thromboembolism with patent foramen ovale: a case report. Cases J. 2009. 2:8358.
9. Kouskov OS, Nichols DJ, O'Hearn DJ. Paradoxical arterial embolism involving both upper extremities in a patient with pulmonary embolism and a patent foramen ovale. Clin Appl Thromb Hemost. 2011. 17:E98–E101.
10. Tanislav C, Puille M, Pabst W, et al. High frequency of silent pulmonary embolism in patients with cryptogenic stroke and patent foramen ovale. Stroke. 2011. 42:822–824.
11. Thomas DV, Bynevelt M, Price R. Paradoxical embolization via a patent foramen ovale following acute pulmonary embolism. Australas Radiol. 2005. 49:501–504.
12. Augustinos P, Ouriel K. Invasive approaches to treatment of venous thromboembolism. Circulation. 2004. 110:9 Suppl 1. I27–I34.
13. Guo S, Roberts I, Missri J. Paradoxical embolism, deep vein thrombosis, pulmonary embolism in a patient with patent foramen ovale: a case report. J Med Case Rep. 2007. 1:104.
14. Dao CN, Tobis JM. PFO and paradoxical embolism producing events other than stroke. Catheter Cardiovasc Interv. 2011. 77:903–909.
15. Konstantinides S, Geibel A, Kasper W, Olschewski M, Blümel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation. 1998. 97:1946–1951.
16. Khairy P, O'Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med. 2003. 139:753–760.
17. Windecker S, Wahl A, Nedeltchev K, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol. 2004. 44:750–758.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download