Abstract
Patent ductus arteriosus (PDA) is a rare clinical finding in adult patients. Considering the increase in cases of PDA discovered incidentally on echocardiograms at young ages, and the life-shortening effect of PDA, it is rare to diagnose PDA in old patients. We report a case of an 80-year-old patient who experienced symptoms of congestive heart failure showed findings suggestive of PDA in echocardiogram and confirmed the diagnosis through a cardiac catheterization and a coronary angiography. After percutaneous occlusion of PDA with an Amplatzer duct occlusion device, symptoms related to congestive heart failure improved.
Functional closure of the ductus arteriosus from vasoconstriction occurs shortly after term birth. Failure of the duct closure between the pulmonary artery and the aorta is called patent ductus arteriosus (PDA).1)
It is estimated that the incidence of PDA is approximately 0.02% to 0.04% in term infants. However, depending on the estimated gestational age, the prevalence in preterm neonates varies from 20% to 60% on the third day of life. PDA accounts for 6% to 11% of all congenital heart defects.2)
Isolated PDA are categorized based on the degree of left to right shunting as mild, moderate and severe PDA which can be subsequently complicated with Eisemengers complex when there is reversal of shunting with progression to pulmonary hypertension. The clinical spectrum of presentation of a PDA may range from a "silent" PDA, one with no clinical manifestations but which is incidentally discovered on echocardiogram, to patients who present with congestive heart failure, pulmonary hypertension, signs of volume overload, endocarditis, atrial fibrillation, or recurrent pneumonia. The average age of death is 35 to 40 years.3) In Korea no patient with PDA over 70 years old has been reported.
We report a first case in Korea of PDA with heart failure in octogenarian diagnosed and successfully occluded.
A 80-year-old man visited the emergency room (ER) due to aggravation of dyspnea for a week. He had diagnosed with hypertension nine years ago and blood pressure had been controlled well. He complained of dyspnea of New York Heart Association (NYHA) IV classification at the time of ER visitation. Though he had a smoking history of 50 pack/years, his pulmonary function test which performed three weeks previously for an orthopedics operation showed normal range of lung function without any evidence of obstructive lung disease. He denied any history of cardiorespiratory problems, but stated that he had suffered dyspnea on exertion for a decade without any functional limitations in his routine activities.
Initial blood pressure was 100/60 mm Hg, heart rate was 90/minute, respiratory rate was 22/minute and body temperature was 37.1℃. During checking of review of systems, he noted dyspnea and sputum but denied fever, cough, chest pain, and nocturnal dyspnea. Upon physical examination, grade II/VI systolic murmur best heard at the left upper sternal border in second intercostal space and the second heart sound was physiologically split, suggesting an absence of significant pulmonary hypertension. Pulses seemed normal, with no evidence of pulse deficit. No diastolic murmurs were heard. Rales and wheezing sounds could be heard in both lung fields but pretibial pitting edema wasn't obvious. Pro-B-type natriuretic peptide was 5603 pg/mL.
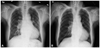
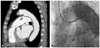
Chest X-ray showed cardiomegaly {cardiothoracic (CT) ratio=0.59} and both pulmonary edema without pleural effusion (Fig. 1A). As a part of his evaluation, a transthoracic echocardiogram (TTE) was performed and found visualized retrograde jet flow at main pulmonary artery, indicating presence of PDA. TTE also showed severe diastolic dysfunction and moderate pulmonary hypertension (right ventricular systolic pressure=57.3 mm Hg) but preserved left ventricular function (ejection fraction of left ventricle=52.6%). End-diastolic volume in M-mode with TTE was 59.2 mm. CT scan of aorta also suggested PDA (Fig. 2A).
Cardiac catheterization and aortic angiogram were done to confirm and check accurately the state of the PDA, proving the presence of a connection between left pulmonary artery and distal aortic arch sized up to 3.1 mm (Fig. 2B). Cardiac catheterization implied PDA, showing a step up from right pulmonary artery to right ventricle (78.7% to 72.9%). Measured Qp/Qs through oxygen saturation was 1.504.
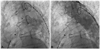
The option of a transcatheter occlusion of PDA had been suggested to the patient to relieve left to right shunt and improve heart failure. Occlusion of PDA with an Amplatzer duct occlusion device was done and no shunt flow was seen in post-procedure aortogram (Fig. 3). After the procedure, CT ratio (0.47) was decreased in the chest X-ray (Fig. 1B) and a follow-up echocardiogram showed no visualized jet flow at the main pulmonary artery indicating successful closure of the PDA. Three days after the closure, a follow-up echocardiogram was done. The end-diastolic dimension was 55.0 mm in M-mode while the right ventricular systolic pressure was 46.0 mm Hg, indicating improvement of heart failure and pulmonary hypertension after the procedure. The patient continues to do well and has no symptom of heart failure or functional limitations without medication including diuretics.
The ductus arteriosus is a vital fetal vascular structure thought to originate from the left sixth aortic arch during embryonic development that connects the main pulmonary artery to the descending aorta. The ductus diverts blood away from the high-resistance, unexpanded fetal circulation into the descending aorta and into the fetal arterial circulation.1) PDA is the failure of the duct closure between the pulmonary artery and aorta and the third most common congenital cardiovascular anomaly, comprising approximately 10% of congenital anomalies or about 1 or 2 in 3000 live births.4) Although most cases of PDA would seem to occur sporadically, multifactorial inheritance is believed to underlie in many cases and these people are thought to possess a genetic predisposition.2)
With improved survival of premature infants who are known to be at risk for PDA and an increase in cases discovered incidentally on echocardiograms performed for another purposes, the apparent incidence of PDA is rising. But presentation in adult life is rare since the lesion is usually diagnosed and closed surgically in infancy or early childhood.3)4)
Clinical manifestations of PDA may vary among people and are dependent on size of the ductus, the age of the patient, the pressure differential across the ductus, and the presence or absence of pulmonary hypertension. Some patients with an underlying PDA may be highly symptomatic, presenting with congestive heart failure, pulmonary hypertension, signs of volume overload, atrial fibrillation, recurrent pneumonia, or other complications known to be associated with PDA. Hemodynamically significant adult PDA has typically been associated with inexorable cardiovascular derangement, including congestive heart failure, infectious endocarditis, and pulmonary hypertension. Others have no signs or symptoms, called "silent". PDA may be discovered only incidentally on an echocardiogram. But even among asymptomatic PDAs who tolerated it for many years without clinical signs or symptoms, patients may become clinically significant with unrepaired PDAs when acquired conditions such as recurrent pneumonia, the development of chronic obstructive pulmonary disease, or the manifestations of valvular or ischemic heart disease are superimposed.2)
The average age of death is 35 to 40 years old and only occasionally can patients survive over 50 years.3) Marquis et al. who studied 804 patients with PDA in Edinburgh between 1940 and 1979 noted that only 37 of them reached the age of 50.5)
In our case, the patient was 80 years old and had had symptoms of heart failure such as dyspnea on exertion. Cough and other symptoms had worsened at the time of ER visitation.
For adults, transcatheter occlusion of the patent ductus is the preferred treatment rather than surgery when possible.6) Percutaneous occlusion using cathether based approaches such as Amplatzer duct occlusion, coil occlusion device and Rashkind umbrella device is the treatment of choice.2) Transcatheter PDA closure is safe and effective in patients with pulmonary hypertension except those with severe pulmonary hypertension.5)6) In one study, 139 patients with PDA were hospitalized and transcatheter closures of PDA were analyzed. After transcatheter closure, the NYHA class and systolic pulmonary arterial pressure was significantly improved.5) In our case, though the patient was in old age and hadn't had severe symptoms, he obviously has improved after percutaneous closure of PDA. However, in adults with large, unfavorably shaped ducts, surgical ligation is the safe treatment of choice and effective alternative choice although calcification of the ductus may increase the technical difficulty of the procedure.7)
We report a case of naive PDA diagnosed at 80 years old with congestive heart failure and successfully closed with Amplatzer duct occlusion. The hemodynamically significant PDA resulted in volume overload of heart and the exhaustion of compensatory mechanisms leading to pulmonary edema and systemic hypoperfusion.8) Pulmonary hypertension and congestive heart failure can be improved by occluding PDA.5)
We suggest that even in the case of PDA in old age, occlusion of shunt should not be dismissed if shunt flow is enough to result in heart failure. Treatment of choice must be decided case by case considering the size of the ductus, the pressure differential across the ductus, and the presence or absence of pulmonary hypertension, but transcatheter occlusion can be the clinician's priority when possible.
Figures and Tables
Fig. 1
Initial chest X-ray shows cardiomegaly. CT ratio was 0.59. Pulmonary edema in both lungs without pleural effusion can be seen (A). The patient's cardiomegaly and pulmonary edema shows improvement after transcatheter closures of patent ductus arteriosus. CT ratio has decreased to 0.47 and symptoms related to heart failure improved without medical treatment including diuretics (B).
References
1. Akintunde AA, Opadijo OG. Case report of a 26 year old primigravida with patent ductus arteriosus (PDA) in heart failure. Afr Health Sci. 2011. 11:138–140.
2. Cassidy HD, Cassidy LA, Blackshear JL. Incidental discovery of a patent ductus arteriosus in adults. J Am Board Fam Med. 2009. 22:214–218.
3. Satoh T, Yanagitani Y, Okano Y. Patent ductus arteriosus with combined valvular disease at age 91. Intern Med. 1997. 36:340–344.
4. Satoh T, Nishida N. Patent ductus arteriosus with infective endocarditis at age 92. Intern Med. 2008. 47:263–268.
5. Zhu XY, Chen HY, Zhang DZ, et al. Effects of transcatheter closure of patent ductus arteriosus in 139 adult patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2009. 37:998–1000.
6. Wang JK, Wu MH, Hwang JJ, Chiang FT, Lin MT, Lue HC. Transcatheter closure of moderate to large patent ductus arteriosus with the Amplatzer duct occluder. Catheter Cardiovasc Interv. 2007. 69:572–578.
7. Harrison DA, Benson LN, Lazzam C, Walters JE, Siu S, McLaughlin PR. Percutaneous catheter closure of the persistently patent ductus arteriosus in the adult. Am J Cardiol. 1996. 77:1094–1097.
8. Giliberti P, De Leonibus C, Giordano L, Giliberti P. The physiopathology of the patent ductus arteriosus. J Matern Fetal Neonatal Med. 2009. 22:Suppl 3. 6–9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download