Abstract
Surgical replacement of the aortic valve is the standard therapy for severe aortic valve stenosis. However, it is generally associated with increased mortality and morbidities in older individuals. Transcatheter aortic valve implantation (TAVI) is a less invasive procedure and has shown similar clinical outcomes as surgical treatment in elderly patients at high risk for conventional surgery. In this report, we describe the first case of TAVI using a CoreValve in Korea. An 84-year-old man with symptomatic severe aortic valve stenosis was successfully treated by transfemoral TAVI. The patient was discharged without any significant complications and remained free of adverse clinical event for a follow-up duration of 6 months.
Aortic stenosis (AS) is the most frequently acquired native valvular disease afflicting the elderly population in Western countries. The prevalence of degenerative AS among the elderly in Korea is also increasing as the Korean population is growing older. Symptomatic severe AS is a lethal disease with a 2-year mortality rate of 50%.1) Until recently, the only definitive treatment of severe AS has been surgical aortic valve replacement. However, accompanying comorbidities in elderly patients are associated with increased procedure-related morbidity and mortality rates. About one third of patients with severe AS and older than 75 years are not referred to a surgeon because of the high surgical risk associated with comorbidities.1)
The development of a catheter-based therapy for heart valve disease, in particular AS, is rapidly evolving. Transcatheter aortic valve implantation (TAVI) allows the aortic valve to be replaced without a sternotomy or cardiopulmonary support. In this case report, we describe the first Korean patient treated by TAVI using a CoreValve (Medtronic, Minneapolis, MN, USA).
An 84-year old male presented with shortness of breath during ordinary daily physical activity {New York Heart Association (NYHA) functional classification class III}. He also experienced syncope 2 months prior to admission to our hospital and intermittent chest pain for 2 weeks leading up to admission. The patient had a past medical history of hypertension and chronic obstructive pulmonary disease. He had been diagnosed with moderate AS 4 years ago and followed up regularly with checkups in the outpatient clinic, and the patient had been prescribed irbesartan, amlodipine, hydrochlorothiazide, atorvastatin. On admission his blood pressure and pulse rate were 150/70 mm Hg and 78/min, while body weight and height were 53 kg and 172 cm. The 12-lead electrocardiogram (ECG) on admission showed sinus rhythm with intermittent premature atrial complexes and findings suggestive of left ventricular hypertrophy with strain signs on lateral leads. Cardiomegaly was noted on the chest X-ray. Transthoracic and transesophageal echocardiography revealed severe degenerative change of aortic valve with heavy calcification (Fig. 1A). The aortic valve area was 0.76 cm2 with a mean systolic pressure gradient of 43 mm Hg and peak systolic pressure gradient of 79 mm Hg compatible with severe AS. The aorta diameter was 21 mm at the aortic annulus, 32 mm at the sinus of valsalva, and 36 mm in the ascending aorta. Left ventricular end diastolic diameter was 52 mm and ejection fraction was 67%. There was mild pulmonary hypertension with an estimated right ventricular systolic pressure of 38 mm Hg. Coronary angiography showed mild coronary artery disease with 50% stenosis at the mid left anterior descending segment. Computed tomography showed diffuse atherosclerotic change of aorta and both external iliac arteries with calcification. The minimal lumen diameter of right and left external iliac arteries was 6 mm and 7 mm, respectively. A pulmonary function test revealed mild obstructive airway disease. The logistic Euroscore was calculated to be 36.1%.
The TAVI procedure was performed under general anesthesia. The vascular access for CoreValve delivery catheter was obtained at the right common femoral artery (CFA) with standard percutaneous access techniques. An 18 Fr introducer sheath was inserted through the right CFA after preparing percutaneous closure using two pre-loaded suture devices (Perclose Proglide, Abbott Vascular, Abbott Park, IL, USA). A pig tail catheter was inserted through the left CFA and positioned at the aortic root for the aortography during the procedure. A temporary pacemaker was placed in the right ventricle via the left femoral vein. A 0.035 inch Amplatz Super Stiff wire (Boston Scientific, Natick, MA, USA) was inserted into the left ventricle through the 18 Fr sheath. Balloon dilation of the stenotic aortic valve was performed with a balloon (diameter 25 mm, length 40 mm, Z-med, NuMED Inc., Hopkinton, NY, USA) under rapid pacing using a temporary pacemaker. Then, a 29 mm CoreValve was deployed at the aortic annulus under angiographic guidance (Fig. 2A). An immediate post-procedural aortogram showed good position of the CoreValve with mild aortic regurgitation (Fig. 2B). Post-procedural transthoracic echocardiography demonstrated a well-functioning CoreValve with a mean systolic pressure gradient of 7 mm Hg and peak systolic pressure gradient of 16 mm Hg (Fig. 1B). The right ventricular systolic pressure decreased from 38 to 31 mm Hg, accompanied by moderate paravalvular aortic regurgitation (grade II/IV). After the procedure, the patient was observed in an intensive care unit for 72 hours with continuous ECG monitoring and temporary pacemaker back-up. No significant conduction abnormalities were observed except for transient nonspecific intraventricular conduction delay with incomplete left posterior hemifascicular block. The patient's symptoms subsequently improved from NYHA class III to class I and was discharged from the hospital at day 8 post procedure without any significant complication. A 3-month follow-up echocardiography revealed no significant interval change from the immediate post procedure echocardiographic findings. The patient remained free from any symptom or any major cardiovascular event for a total follow-up period of 6 months.
This is the first case of TAVI in Korea using a CoreValve. The current indications for TAVI with CoreValve are the following: 1) symptomatic, severe, and native aortic valve stenosis; 2) aortic annular dimensions between 18 and 27 mm and an ascending aortic diameter of less than 45 mm; 3) age ≥80 years, surgical risk calculated with logistic EuroSCORE >20%,2)3) or age ≥65 years plus at least 1 of the following risk factors: liver cirrhosis, respiratory failure, pulmonary hypertension, previous cardiac surgery, right ventricular failure, hostile thorax (such as radiation, burns, previous thoracic pleurodesis, or multiple thoracotomies), severe connective tissue disease, cachexia, and porcelain aorta. Exclusion criteria for TAVI comprised any of the following: 1) sepsis or active endocarditis, 2) hypersensitivity or contraindication to acetylsalicylic acid or clopidogrel, 3) bleeding diathesis or coagulopathy, 4) recent myocardial infarction or cerebrovascular event, 5) the presence of left ventricular or atrial thrombus, 6) previous aortic valve replacement, and 7) a secondary progressive disease with an expectancy of life of less than 1 year.4) The patient was 84 years old and had symptomatic severe aortic valve stenosis complying with the indications for TAVI, and no exclusion criteria were present.
Currently, two types of aortic valves are commercially available for percutaneous implantation, which are the balloon-expandable Edwards-Sapien Transcatheter Heart Valve (Edwards, Lifesciences, Irvine, CA, USA) and the self-expandable CoreValve.5) In the PARTNER A trial, a randomized controlled trial comparing TAVI using Edwards Sapien valve and surgical aortic valve replacement in high-risk patients with severe AS, both treatments showed similar rates of survival and major stroke rate. However, all stroke and vascular complication rates were higher in the TAVI group, whereas major bleeding occurred more frequently in the surgery group.6) The PARTNER B trial compared TAVI and medical treatment for clinical outcomes in patients who could not undergo surgery. Although TAVI had major complications of stroke, vascular complications, and major bleeding, but could lower all-cause mortality about 20% over standard medical treatment. A recent published Italian multicenter CoreValve registry study reported sustained clinical and functional cardiovascular benefits in high-risk patients with symptomatic AS up to a 3-year follow-up period.
A randomized clinical trial comparing TAVI using CoreValve and surgical treatment is still ongoing. Although there have been no randomized clinical trials comparing the two aortic valve systems, both systems appear to be similar in procedural success rates and clinical outcomes according to various registry data. In most recent studies, the procedural success and 30-day survival rates range over 90%.8) Stroke is currently the major concern after TAVI, and so far stroke rates have been reported to be <5%.8)9) Generally, the Core-Valve device is associated with less hemodynamic instability during deployment. Therefore, it is released gradually by adjusting its position and potentially retrievable if positioned incorrectly. By contrast, it is difficult to adjust the Sapien valve position during deployment. The risk of acute coronary obstruction by a displaced native valve leaflet may be lower with CoreValve. Significant atrioventricular (AV) conduction system abnormalities requiring permanent pacemaker implantation have been reported after TAVI.10) As the stent containing the valve courses through the interventricular septum below the aortic valve, injury to the AV conduction system may be associated with new left bundle or complete heart block. The need for a permanent pacemaker appears to be higher with the Core-Valve (range 9-36%) than with the Sapien valve (range 3-12%).9)10) This may be due to the fact that the stent of the CoreValve is longer than that of the Sapien valve and has more contact with the left ventricular outflow tract.9)10) In our patient, new nonspecific intraventricular conduction delay with incomplete left posterior hemifascicular block was noted after TAVI. However, it did not require pacemaker implantation. It is generally recommended to avoid low implantation of the valve and to monitor ECGs for at least 3 days after TAVI procedures. Although the device profile has been decreased to 18 Fr, the major vascular complication rate remains relatively high (>10% in most instances).5)8) A careful estimation of the diameter, tortuosity and calcification of the iliofemoral arteries by angiography, computed tomography, or intravascular ultrasound in borderline case seems to be important in reducing such vascular complications.11) In this case, the diameters of the iliac and common femoral arteries were larger than 6 mm and there was no problem with engaging the catheter through the iliofemoral artery to aorta. In the presence of inadequate transfemoral access routes, other vascular access such as transaxillar or direct aortic access needs to be considered. In contrast to the Sapien valve, the CoreValve is not available for transapical approach. In our patient, paravalvular leakage of a moderate degree was observed after the procedure. The long-term fate of this regurgitation is not well known. After the procedure, the patient's symptoms were markedly improved from NYHA class III to class I. Durability and long-term safety of the catheter-based aortic valves need to be clarified in further large-scale clinical trials with long-term follow-ups.9)12)13)
Figures and Tables
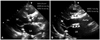
Fig. 1
Transthoracic echocardiogram with a parasternal long axis view of the aortic valve. Sclerocalcified stenotic aortic valve before TAVI (arrow, A) and implanted CoreValve after TAVI (arrowheads, B). AV: aortic valve, LA: left atrium, LV: left ventricle, RV: right ventricle, TAVI: transcatheter aortic valve implantation.
References
1. Gonçalves A, Marcos-Alberca P, Almeria C, et al. Quality of life improvement at midterm follow-up after transcatheter aortic valve implantation. Int J Cardiol. 2011. [Epub ahead of print].
2. Walther T, Kempfert J, Rastan A, et al. Transapical aortic valve implantation at 3 years. J Thorac Cardiovasc Surg. 2012. 143:326–331.
3. Ben-Dor I, Gaglia MA Jr, Barbash IM, et al. Comparison between Society of Thoracic Surgeons score and logistic EuroSCORE for predicting mortality in patients referred for transcatheter aortic valve implantation. Cardiovasc Revasc Med. 2011. 12:345–349.
4. Ong SH, Beucher H, Mueller R, Gerckens U, Boekstegers P. Percutaneous double-valve interventions for aortic stenosis and pure mitral regurgitation. Am J Cardiol. 2011. 108:893–895.
5. Webb J, Cribier A. Percutaneous transarterial aortic valve implantation: what do we know? Eur Heart J. 2011. 32:140–147.
6. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010. 363:1597–1607.
7. Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the Placement of Aortic Transcatheter Valves (PARTNER) Trial (Cohort B). Circulation. 2012. 125:1102–1109.
8. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011. 364:2187–2198.
9. Rodés-Cabau J. Progress in transcatheter aortic valve implantation. Rev Esp Cardiol. 2010. 63:439–450.
10. Toutouzas K, Michelongona A, Synetos A, Latsios G, Tsioufis C, Stefanadis C. Atrioventricular block 9 days after transcatheter aortic valve implantation. Int J Cardiol. 2011. 151:112–114.
11. Chu MW, Falk V, Mohr FW, Walther T. Percutaneous endoscopic transapical aortic valve implantation: three experimental transcatheter models. Interact Cardiovasc Thorac Surg. 2011. 13:251–256.
12. Sarkar K, Ussia GP, Tamburino C. Transcatheter aortic valve implantation for severe aortic regurgitation in a stentless bioprosthetic valve with the Core Valve revalving system-technical tips and role of the Accutrak system. Catheter Cardiovasc Interv. 2011. 78:485–490.
13. Azadani AN, Jaussaud N, Ge L, Chitsaz S, Chuter TA, Tseng EE. Valve-in-valve hemodynamics of 20-mm transcatheter aortic valves in small bioprostheses. Ann Thorac Surg. 2011. 92:548–555.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download