Abstract
Background and Objectives
The relationship between the positive remodeling (PR) of a coronary artery and plaque composition has been studied only in a relatively small number of study population or non-culprit lesion. We evaluated the association between coronary plaque composition and coronary artery remodeling in a relatively large number of culprit lesions.
Subjects and Methods
The study population consisted of 325 consecutive patients with coronary artery disease that underwent intravascular ultrasound-virtual histology examination in a culprit lesion. The remodeling index (RI) was calculated as the lesion external elastic membrane (EEM) area divided by the average reference EEM area.
Results
The lesions with PR (RI>1.05, n=97, mean RI=1.19±0.12) had a higher fibrous volume/lesion length (3.85±2.12 mm3/mm vs. 3.04±1.79 mm3/mm, p=0.003) and necrotic core volume/lesion length (1.26±0.89 mm3/mm vs. 0.90±0.66 mm3/mm, p=0.001) than those with negative remodeling (NR) (RI<0.95, n=132, mean RI=0.82±0.09). At the minimal luminal area site, the lesions with PR had a higher fibrous area (5.81±3.17 mm2 vs. 3.61±2.30 mm2, p<0.001), dense calcified area (0.73±0.69 mm2 vs. 0.46±0.43 mm2, p=0.001), and necrotic core area (1.93±1.33 mm2 vs. 1.06±0.91 mm2, p<0.001) than those with NR. RI showed significant positive correlation with fibrous volume/lesion length (r=0.173, p=0.002), necrotic core volume/lesion length (r=0.188, p=0.001), fibrous area (r=0.347, p<0.001), fibrofatty area (r=0.111, p=0.036), dense calcified area (r=0.239, p<0.001), and necrotic core area (r=0.334, p<0.001). Multivariate analysis showed that the independent factor for PR was the necrotic core volume/lesion length (beta=0.130, 95% confidence interval; 0.002-0.056, p=0.037) over the entire lesion.
Vascular remodeling refers to the compensatory change of vessel size responded to plaque growth by vascular expansion (positive remodeling, PR) or constriction (negative remodeling, NR). Pathologic study confirmed that plaque growth with PR did not affect the lumen area until the atherosclerotic plaque reached 40% of the internal elastic lamina areas.1) However, in vivo intravascular ultrasound (IVUS) studies showed that NR is also observed in 15-50% of stenotic lesions in a human coronary artery2)3) and that the target lesions in patients with acute coronary syndrome (ACS) more frequently exhibited PR and a large plaque area, whereas patients with stable angina more frequently showed intermediate or NR and a smaller plaque area.4)5)
Varnava et al.6) have reported that a PR lesion has higher lipid contents and a macrophage count, both markers of plaque vulnerability in a necropsy study. However, a histologic study has limitations including tissue shrinkage during fixation and postmortem contraction of arteries. Although an IVUS study has shown an in vivo coronary plaque morphology, it also has significant limitations in assessing plaque morphology, especially in discriminating fibrous from fatty tissue.7)8) On the other hand, a recently developed virtual histology (VH)-IVUS provides a method of accurate in vivo analysis of coronary plaque using radiofrequency spectral analysis identifying the fibrous, fibro-fatty, dense calcium and necrotic cores in the coronary plaque in a coronary artery. It has been shown to have a 93-97% ex vivo and 87-92% in vivo accuracy for specific tissue composition.9)10) Recently, a few studies using VH reported conflicting data about plaque composition according to the remodeling index (RI).11-13) These studies conducted only in relatively small numbers of the study population or a non-culprit lesion. We sought to evaluate the association between coronary plaque composition and coronary artery remodeling with VH-IVUS analysis in a relatively large number of culprit lesions.
From July 2006 to July 2008, a total of 325 consecutive patients who underwent coronary angiography (CAG) and/or percutaneous coronary intervention (PCI) and VH-IVUS study were enrolled. The exclusion criteria included severe calcified and tortuous vessels that impossible to pass an IVUS catheter, a history of PCI or coronary artery bypass surgery, hemodynamically unstable patients and patient who refused the study. Patient demographics and laboratory data, including a fasting lipid profile and serum glucose, were obtained before the IVUS study. Written informed consent was obtained from all patients, and the study was approved by the hospital ethics committee of a University Hospital.
All patients received aspirin 300 mg and clopidogrel 300-600 mg, and 120 IU/kg of unfractioned heparin intravenously before CAG. CAG was done by the femoral or radial approach using a 6 or 7 Fr guiding catheter and 0.014-inch standard or extra-support coronary guidewires. The definitions of a culprit lesion were: prespecified 1) the site of acute coronary occlusion or, 2) for non-occluded arteries, the site of the greatest narrowing within an angiographically significant stenosis corresponding to the electrocardiographic changes.
The VH-IVUS examination was performed on the culprit lesion with a dedicated 20-MHz, 2.9 F monorail, electronic Eagle Eye Gold IVUS catheter (Volcano Therapeutics, Rancho Cordova, CA, USA) and VH-IVUS console (Volcano Therapeutics, Rancho Cordova, CA, USA) during the CAG after the intracoronary administration of 100 to 200 µg nitroglycerin. The VH-IVUS image was recorded on a DVD-ROM for off-line analysis later.
Qualitative and quantitative analyses of gray scale IVUS images were performed according to the criteria of the American College of Cardiology's Clinical Expert Consensus Document on IVUS.14) The proximal and distal references were defined as the site with the largest lumen proximal distal to a stenosis but within the same segment (usually within 10 mm of the stenosis with no major intervening branches), respectively.
An external elastic membrane (EEM) cross-sectional area (CSA) was measured with customized software (IVUS Lab., Volcano Therapeutics, Rancho Cordova, CA, USA). The RI was calculated as the lesion EEM CSA divided by the average reference EEM CSA. In this study, PR was defined as RI >1.05 and NR as RI <0.95. Values in between were considered intermediate or no remodeling.
These analyses were done on the target lesion with customized software (IVUS Lab.; Volcano Therapeutics, Rancho Cordova, CA, USA) by an examiner who was unaware of the gray scale IVUS results. For both the lumen and the media-adventitia interface, an automatic border detection was done at the predefined lesion segment. Then, the border detection was manually corrected again in the lesion after the automatic border detection. Border detection required the agreement of two independent experienced cardiologists. Disagreements were reviewed by a third cardiologist. After confirming the border detection, the software would automatically calculate and show the results. For each frame, histologic findings were expressed in colors: (green for fibrous, green-yellow for fibro-fatty, white for dense calcified, and red for necrotic core areas) (Fig. 1). The predictive accuracy of this method with tissue mapping has been validated.9) The area (mm2) and percent area of each tissue component of plaque were analyzed at the minimal luminal area site, and the volume (mm3) and percent volume of each tissue component of plaque were evaluated at the full segment of a culprit lesion. Volume was divided by lesion length to adjust for a different lesion length of each patient and then described as corrected volume (mm3/mm).
All analyses were performed with Statistical Package for the Social Sciences (SPSS) (version 18.0; SPSS Inc., Chicago, IL, USA). All data are expressed as mean±standard deviation for continuous variables and as a percentage ratio for categorical variables. Clinical characteristics in the positive and NR groups were compared using the independent t-test for continuous variables and chi-square test for categorical variables. The Pearson correlation coefficient was used to measure the association between RI and each variable. A multiple regression analysis with a backward stepwise method was performed to evaluate independent predictors of plaque compositions for PR. A p<0.05 was considered statistically significant.
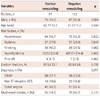
Of 325 patients, 97 patients had PR (RI >1.05, mean RI=1.19±0.12), and 132 patients had NR (RI <0.95, mean RI=0.82±0.09). The PR group had a higher prevalence of ST elevation myocardial infarction (37.1% vs. 22.0%, p=0.017) and non ST elevation ACS (19.6% vs. 16.6%, p=0.017) than NR group. There were no other differences in baseline patient and lesion characteristics (Table 1).
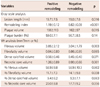
At the entire segment of the culprit lesion, the lesions with PR had higher corrected fibrous volume (3.85±2.12 mm3/mm vs. 3.04±1.79 mm3/mm, p=0.003) and corrected necrotic core volume than those with NR (Table 2, Fig. 2).
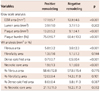
At the minimal lumen area (MLA) site, the lesions with PR had a higher plaque burden (75.6±6.7% vs. 68.4±10.2%, p<0.001), fibrous area (5.81±3.17 mm2 vs. 3.61±2.30 mm2, p<0.001), dense calcified area (0.73±0.69 mm2 vs. 0.46±0.43 mm2, p=0.001), and necrotic core area (1.93±1.33 mm2 vs. 1.06±0.91 mm2, p<0.001) than those with NR (Table 3).
Remodeling index showed significant positive correlations with corrected fibrous volume (r=0.173, p=0.002), corrected necrotic core volume (r=0.188, p=0.001) (Fig. 3), fibrous area (r=0.347, p<0.001), fibrofatty area (r=0.111, p=0.036), dense calcified area (r=0.239, p<0.001), and necrotic core area (r=0.334, p<0.001) (Fig. 4).
A linear regression analysis with a backward method showed that the independent factor for PR was the corrected necrotic core volume (beta=0.130, 95% confidence interval; 0.002-0.056, p=0.037) at the culprit lesion (Table 4).
The main finding of this in vivo study with VH-IVUS was that PR was associated with the necrotic core volume at the culprit lesion.
Studies about the correlation between plaque composition analyzed by VH-IVUS and RI showed conflicting results. Fujii et al.11) demonstrated that PR occurs in a lesion with more fibrofatty plaque, Rodriguez-Granillo et al.12) reported that RI is positively correlated with lipid core and inversely with fibrous tissue, and Surmely et al.13) reported that lesions with PR have less necrotic core percent area at the minimal luminal diameter site and no differences of plaque compositions in the entire culprit lesion. However, Fujii et al.11) studied a small number of study subjects including non-culprit lesions and used a different definition of RP (RI >1.0). Rodriguez-Granillo et al.12) studied non-significant lesions (<50% diameter stenosis by angiography). Although Surmely et al.13) studied culprit lesions, they divided the study subjects into two groups according to RI: PR (RI ≥1.05) and intermediate/NR (RI <1.05). The different study subjects and method can explain the different results from our study. Furthermore, the IVUS catheter in our study (20-MHz, electronic Eagle Eye Gold IVUS catheter, Volcano Therapeutics, Rancho Cordova, CA, USA) was different from that in Fujii et al.11) and Rodriguez-Granillo et al.12) studies (30-MHz, mechanically rotating IVUS catheter, Boston Scientific, Santa Clara, CA, USA). Postmortem pathologic studies for coronary artery disease demonstrate that plaque with PR had a higher lipid content and characteristics of vulnerable plaque.6)15) The present study showed different results from the previous study with VH-IVUS, but corresponded with necropsy study in terms of plaque compositions.
Coronary plaque with PR is more frequently found in patients with ACS and vulnerable plaque, which is main cause of ACS, usually demonstrated PR. In this study, the PR group had a higher prevalence of ACS in clinical presentation. Although the plaque vulnerability characterized by thin-cap fibroatheroma did not evaluate in this study, the independent predictor of PR was the necrotic core volume in the culprit lesion that is one of main characterizations of vulnerable plaque, not the necrotic core area at the MLA site. This result is compatible with previous one that demonstrated the association between necrotic core volume and the clinical presentation of ACS.16)
The current study had some limitations. First, this study was a single-center, retrospective study. However, study subjects were consecutively enrolled and a relatively large population that divided into the PR and NR group. Second, severe calcified and tortuous vessels that impossible to pass an IVUS catheter and heavily calcified lesions that cannot detect the media-adventitia interface were excluded and may have led to selection bias. Finally, the current VH-IVUS tree is not able to differentiate intraluminal thrombus from other plaque components.17) The thrombus may be misclassified as fibrous plaque (fibrofatty dependent on age), proportionally increasing this plaque component at the expense of the others.18) Furthermore, some investigators raised a concern about the accuracy of VH-IVUS in detecting plaque composition.19)20) However, no single method is perfect in the accuracy and VH-IVUS is currently used in many clinical studies to detect plaque composition.
In conclusion, this in vivo study demonstrated that PR is associated with a lipid core in a culprit lesion and supports the results of previous postmortem studies that lipid accumulation in a culprit lesion is related with vascular expansion in atherogenesis.
Figures and Tables
Fig. 1
Cross-sectional images of virtual histology (VH) intravascular ultrasound (IVUS) from distal to proximal within a same lesion in A (patient with NR) and B (patient with PR). VH-IVUS images in patient with PR (B) shows large amount of necrotic core area throughout the entire lesion length. EEM: external elastic membrane, CSA: cross sectional area.

Fig. 2
Volumetric analyses over the entire lesion segment. Fibrous plaque was larger in lesions with positive remodeling (PR) than in lesions negative remodeling (NR) (A). Necrotic core plaque was larger in lesions with PR than in lesions NR (B).

Fig. 4
Correlations of remodeling index and (A) fibrous area, (B) fibrofatty area, (C) dense calcium area, (D) necrotic core area at the minimal luminal area site.

References
1. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987. 316:1371–1375.
2. Mintz GS, Kent KM, Pichard AD, Satler LF, Popma JJ, Leon MB. Contribution of inadequate arterial remodeling to the development of focal coronary artery stenoses. An intravascular ultrasound study. Circulation. 1997. 95:1791–1798.
3. Hirose M, Kobayashi Y, Mintz GS, et al. Correlation of coronary arterial remodeling determined by intravascular ultrasound with angiographic diameter reduction of 20% to 60%. Am J Cardiol. 2003. 92:141–145.
4. Nakamura M, Nishikawa H, Mukai S, et al. Impact of coronary artery remodeling on clinical presentation of coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol. 2001. 37:63–69.
5. Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation. 2000. 101:598–603.
6. Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002. 105:939–943.
7. Hiro T, Leung CY, De Guzman S, et al. Are soft echoes really soft? Intravascular ultrasound assessment of mechanical properties in human atherosclerotic tissue. Am Heart J. 1997. 133:1–7.
8. Jeremias A, Kolz ML, Ikonen TS, et al. Feasibility of in vivo intravascular ultrasound tissue characterization in the detection of early vascular transplant rejection. Circulation. 1999. 100:2127–2130.
9. Nasu K, Tsuchikane E, Katoh O, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006. 47:2405–2412.
10. Nair A, Margolis MP, Kuban BD, Vince DG. Automated coronary plaque characterisation with intravascular ultrasound backscatter: ex vivo validation. EuroIntervention. 2007. 3:113–120.
11. Fujii K, Carlier SG, Mintz GS, et al. Association of plaque characterization by intravascular ultrasound virtual histology and arterial remodeling. Am J Cardiol. 2005. 96:1476–1483.
12. Rodriguez-Granillo GA, Serruys PW, Garcia-Garcia HM, et al. Coronary artery remodelling is related to plaque composition. Heart. 2006. 92:388–391.
13. Surmely JF, Nasu K, Fujita H, et al. Association of coronary plaque composition and arterial remodelling: a virtual histology analysis by intravascular ultrasound. Heart. 2007. 93:928–932.
14. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001. 37:1478–1492.
15. Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002. 105:297–303.
16. Bae JH, Kwon TG, Kim KH, Hyun DW, Kim KY, Kim DS. In-vivo coronary plaque composition in patients with acute coronary syndrome: a virtual histology intravascular ultrasound study. Korean Circ J. 2007. 37:437–442.
17. Carlier SG, Mintz GS, Stone GW. Imaging of atherosclerotic plaque using radiofrequency ultrasound signal processing. J Nucl Cardiol. 2006. 13:831–840.
18. Frutkin AD, Mehta SK, McCrary JR, Marso SP. Limitations to the use of virtual histology-intravascular ultrasound to detect vulnerable plaque. Eur Heart J. 2007. 28:1783–1784.
19. Granada JF, Wallace-Bradley D, Win HK, et al. In vivo plaque characterization using intravascular ultrasound-virtual histology in a porcine model of complex coronary lesions. Arterioscler Thromb Vasc Biol. 2007. 27:387–393.
20. Alfonso F, Hernando L. Intravascular ultrasound tissue characterization. I like the rainbow but...what's behind the colours? Eur Heart J. 2008. 29:1701–1703.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download