Abstract
Background and Objectives
The effects of fenofibrate on C-reactive protein (CRP) are under debate. We investigated the effect of fenofibrate on CRP levels and the variables determining changes.
Subjects and Methods
This case-control study enrolled 280 hypertriglyceridemic patients who were managed either with 200 mg of fenofibrate (Fenofibrate group, n=140) or with standard treatment (comparison group, n=140). CRP levels were measured before and after management for 2 months.
Results
CRP levels decreased in both the fenofibrate (p=0.003) and comparison (p=0.048) groups. Changes in CRP levels were not significantly different between the two groups (p=0.27) and were negatively associated with baseline CRP levels (r=-0.47, p<0.001). In patients with a baseline CRP level ≥1 mg/L, CRP levels also decreased in both groups (p=0.000 and p=0.001 respectively), however, more in the fenofibrate group than in the comparison group (p=0.025). The reduction of CRP was associated with higher baseline CRP levels (r=-0.29, p=0.001), lower body mass index (BMI, r=0.23, p=0.007), and fenofibrate therapy (r=0.19, p=0.025). CRP levels decreased more in the fenofibrate group than in the comparison group in patients with a BMI ≤26 kg/m2 with borderline significance (-1.21±1.82 mg/L vs. -0.89±1.92 mg/L, p=0.097). In patients with a high density lipoprotein-cholesterol level <40 mg/dL, CRP levels were reduced only in the fenofibrate group (p=0.006).
Fibrate is a peroxisome proliferator-activated receptor α agonist and is widely used to decrease triglyceride and to increase high density lipoprotein-cholesterol (HDL-C).1) Several large-scale studies have investigated the effect of fibrate in the prevention of cardiovascular events.2-6) However, the results are contentious. Inflammatory processes play an important role not only in the pathogenesis of atherosclerosis but also in the occurrence of acute coronary syndromes.7) C-reactive protein (CRP) is a prototype of inflammatory markers and high levels of CRP are associated with an increased risk for cardiovascular diseases.8) Several studies have investigated the effect of fibrate on CRP levels.9-25) However, most studies had limitations in the study design and the results were inconsistent. In addition, most studies enrolled too few patients for subgroup analysis. The aims of this study were to evaluate the effect of fenofibrate on CRP levels in hypertriglyceridemic patients compared with that of the well-matched comparison group and to explore variables affecting CRP levels in a relatively large number of patients.
This retrospective case-control enrolled 280 patients with a triglyceride level ≥200 mg/dL. Exclusion criteria were 1) new onset diseases that influence lipid levels, such as diabetes mellitus, infectious diseases, or other endocrinologic diseases within 3 months, 2) aspartate aminotransferase or alanine aminotransferase levels ≥3-fold the upper normal limit, 3) medications within 3 months that affect lipid levels, and 4) baseline and follow-up CRP levels ≥10 mg/L. Patients were divided into two groups: the comparison group was managed with general measures (n=140) and the fenofibrate group was treated with 200 mg of fenofibrate (n=140). General measures included a low-calorie and a low-fat diet and aerobic exercise. A part of these data was published previously.23)
Concentrations of CRP and lipids were measured before and after management for 2 months. After overnight fasting, blood samples were obtained. Concentrations of total cholesterol and triglyceride were determined by the enzymatic method using an automatic analyzer (Model 7150, Hitachi, Tokyo, Japan). The concentration of HDL-C was measured by the direct method using an automatic analyzer. The concentration of high sensitivity CRP was determined by nephelometer method using an N High Sensitivity CRP kit (Dade Behring Marburg GmbH, Marburg, Germany).
Data are expressed as mean±SD. Statistical analysis was performed using the Social Package for the Social Sciences (SPSS V9.0K, SPSS Inc., Chicago, IL, USA). For CRP and triglyceride, the Wilcoxon signed-rank test was used to compare concentrations before and after therapy, and the Mann-Whitney U test was used to evaluate differences between groups. For other variables, the paired t-test was used to compare the concentrations before and after medication, and Student's t-test was used to evaluate differences between groups. CRP and triglyceride were log-transformed and the relationships between parameters were analyzed using Pearson's correlation method. Stepwise linear regression method was used to obtain independent variables. The distribution of discrete variables was analyzed using the χ2 test. Two-tailed null hypotheses of no difference were rejected if p were less than 0.05.
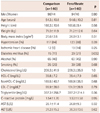
Baseline demographic and clinical characteristics were similar between the two groups except for the number of patients with diabetes mellitus (p=0.032). Baseline CRP levels and lipid profiles were also similar (Table 1).
C-reactive protein levels decreased in both the fenofibrate (from 1.53±1.58 to 1.29±1.44 mg/L, p=0.003) and comparison (from 1.54±1.70 to 1.40±1.44 mg/L, p=0.048) groups (Table 2). Changes in CRP levels were not significantly different between the two groups (-0.24±1.56 mg/L vs. -0.14±1.69 mg/L, p=0.27). In all patients (n=280), CRP levels decreased more in patients with higher baseline CRP levels than in those with lower levels (r=-0.47, p<0.001) (Table 3, Fig. 1). Fenofibrate increased HDL-C level (p=0.000) and decreased total cholesterol (p=0.026), nonHDL-C (p=0.000), and triglyceride (p=0.000) levels compared with the comparison group. There were no significant changes in low density lipoprotein-cholesterol levels (p=0.81) between the two groups.
In patients with a baseline CRP level ≥1 mg/L, CRP levels decreased in both the fenofibrate (n=69, from 2.55±1.73 to 1.76±1.74 mg/L, p=0.000) and comparison (n=72, from 2.49±1.92 to 1.83±1.36 mg/L, p=0.001) groups (Table 2). CRP levels fell more in the fenofibrate group than in the comparison group (-0.79±1.90 mg/L vs. -0.66±1.77 mg/L, p=0.025). In these patients (n=141), CRP levels decreased more in patients with higher baseline CRP levels (r=-0.29, p=0.001) (Fig. 1), lower body mass index (BMI, r=0.23, p=0.007) (Fig. 2), fenofibrate therapy (r=0.19, p=0.025), and lower body weight (r=0.17, p=0.040) (Table 3). Among these variables, higher baseline CRP levels, lower BMI, and fenofibrate therapy were independent in the stepwise linear regression analysis. The association of changes in CRP levels with BMI was found only in the fenofibrate group (r=0.28, p=0.022) and not in the comparison group (r=0.17, p=0.15) (Fig. 2). In patients with a baseline CRP level <1 mg/L, CRP levels did not change in both the fenofibrate (n=71, p=0.11) and comparison (n=68, p=0.20) groups (Table 2).
In all patients (n=280), baseline CRP levels were higher in patients with a BMI >26 kg/m2 than in those with a BMI ≤26 kg/m2 (1.63±1.54 mg/L vs. 1.47±1.71 mg/L, p=0.014). However, when patients with a baseline CRP level <1 mg/L were excluded, there were no significant differences between the two groups (2.37±1.64 mg/L vs. 2.67±1.98 mg/L, p=0.59). In patients with a CRP level ≥1 mg/L and a BMI ≤26 kg/m2, CRP levels decreased in both the comparison and fenofibrate groups (p=0.004 and p=0.000, respectively) and more in the fenofibrate group than in the comparison group with a borderline significance (-1.21±1.82 mg/L vs. -0.89±1.92 mg/L, p=0.097) (Table 2). In patients with a baseline CRP level ≥1 mg/L and a BMI >26 kg/m2, CRP levels tended to decrease in both groups (p=0.064 and p=0.057, respectively). However, these changes were not significantly different between the two groups (p=0.63).
In patients with a baseline HDL-C level <40 mg/dL, CRP levels decreased in the fenofibrate group (n=83, p=0.006) and not in the comparison group (n=79, p=0.12) (Table 2). However, changes in CRP levels were not significantly different between the two groups (-0.30±1.42 vs. -0.17±1.83, p=0.41). In patients with a baseline HDL-C level ≥40 mg/dL, CRP levels did not change in both the fenofibrate (n=57, p=0.15) and comparison (n=61, p=0.27) groups.
The present study demonstrated that fenofibrate had a small but a significant anti-inflammatory effect in hypertriglyceridemic patients with high risk for cardiovascular diseases and not severely overweight. To our knowledge, this is the first report to evaluate the effect of fibrate on CRP levels by baseline CRP levels and obesity.
In the present study, patients with diabetes mellitus were included more in the fenofibrate group than in the comparison group (p=0.032). Patients with diabetes mellitus had higher CRP levels than those without diabetes mellitus (2.44±2.23 mg/L vs. 1.37±1.45 mg/L, p=0.001).26) However, changes in CRP levels were not different (0.34±2.08 mg/L vs. 0.22±1.40 mg/L, p=0.35) in the fenofibrate group and the number of patients with diabetes mellitus was relatively small (n=29). Therefore, we believe that this difference did not influence the results.
In all patients, CRP levels decreased in both the comparison and fenofibrate groups. However, changes in CRP levels were not significantly different between the two groups. This finding suggests that the reduction of CRP levels was not by fenofibrate therapy but by general measures.
As changes in CRP levels were closely associated with baseline CRP levels, we divided patients into two subgroups according to a median value of baseline CRP levels. In patients with a CRP level ≥1 mg/L, fenofibrate decreased CRP levels more compared with general measures. This finding suggests that fenofibrate may have an anti-inflammatory effect only in patients with high risks for cardiovascular diseases.
Several studies have investigated the effect of fibrate on CRP levels.9-25) However, most studies did not have a comparison group9-11) or a comparison of fibrate with statins.12-16) In several studies with a comparison group, there were differences in the clinical characteristics and baseline CRP levels.17-19) In the present study, CRP levels decreased not only in the fenofibrate group but also in the comparison group. The reduction was more pronounced in patients with higher CRP levels than in those with lower CRP levels. Since previous studies enrolled high-risk patients with diabetes mellitus, metabolic syndrome, or dyslipidemia, baseline CRP levels were often high. Most of these studies report that fibrate markedly reduces CRP levels. Therefore, it is critical to include a well-matched comparison group to avoid false-positive results and the data from these studies must be interpreted with caution.
In well-designed studies, fibrate markedly reduced CRP levels in several studies20-22) and did not in other studies.23-25) In a retrospective analysis of the Bezafibrate Infarction Prevention (BIP) study which included 3122 patients with chronic coronary heart diseases, CRP levels increased by 3.0% in bezafibrate-treated patients and by 3.7% in the comparison group after 2 years.24) In subgroup analysis of the Fenofibrate Intervention and Event Lowering in Diabetes study which included 170 patients with diabetes mellitus, fenofibrate failed to decrease CRP levels.25) In contrast, several studies with a relatively small number of patients have reported that fibrate reduces CRP levels by 25-51%.20-22) In the present study, fenofibrate significantly reduced CRP levels in patients with a baseline CRP ≥1 mg/L, but not in all patients. The wide range of baseline CRP levels may partially explain the inconsistency of the previous studies.
We excluded patients with baseline and follow-up CRP levels ≥10 mg/L in order to rule out the possibility of inflammation from other causes.27) Most studies did not include these exclusion criteria. Very high baseline and follow-up CRP levels may overestimate and underestimate the effects of fibrate, respectively.
In earlier large-scale studies, fibrate reduced cardiovascular events in patients with high nonHDL-C levels and in patients with coronary artery diseases and low HDL-C levels.2)3) In recent studies, fenofibrate has failed to reduce the primary endpoints.4-6) However, fenofibrate consistently reduced cardiac events in a subgroup with low HDL-C and high triglyceride levels.4-6) In the BIP study, high CRP levels were associated with low HDL-C and high triglyceride levels.4) Therefore, fenofibrate may be effective only in patients who have high CRP levels. In the present study, the effect of fenofibrate was evident in patients with high baseline CRP levels. This finding suggests that baseline CRP levels may be useful to determine whether fibrate is prescribed to a patient or not. For example, statin was more cardioprotective in patients with high CRP levels than in those with low CRP levels.28) Further studies are needed to confirm this hypothesis.
The present study showed a very interesting finding that was previously unknown. The reduction of CRP level was positively associated with BMI (r=0.23, p=0.007) (Fig. 2) in patients with a baseline CRP ≥1 mg/L. Fenofibrate decreased CRP levels more in patients with a BMI ≤26 kg/m2 than in patients with a BMI >26 kg/m2, although it did not reach the statistical significance (p=0.097). This finding suggests that fenofibrate can modify the inflammatory process from the metabolic origin but not that from the obesity. It has been reported that CRP level and the effect of fibrate on it are dependent on genetic polymorphisms.29) In addition, this study showed that the obesity could also influence the effect of fibrate CRP levels.
There are several limitations in this study. This study was performed at a single hospital in Korea. Patients with diabetes mellitus were more frequent in the fenofibrate group than in the comparison group. This study enrolled an adequate number of patients for most analyses. However, the subgroup analysis according to body weight showed a borderline significance due to the small number of patients. This finding needs to be confirmed in more large scale studies.
In conclusion, the present study has shown that fenofibrate has a small but significant anti-inflammatory effect in selected high-risk patients with high inflammatory conditions and without severe overweight and/or with low HDL-C.
Figures and Tables
Fig. 1
The relation between baseline C-reactive protein (CRP) levels and changes in CRP levels in all patients (A), patients with a baseline CRP level ≥1 mg/L (B), and patients with a baseline CRP level <1 mg/L (C).

Fig. 2
The relation between changes in C-reactive protein (CRP) levels and body mass index in patients with a baseline CRP level ≥1 mg/L. A: all patients. B: the comparison group. C: the fenofibrate group.

References
1. Shah A, Rader DJ, Millar JS. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis. 2010. 210:35–40.
2. Frick MH, Elo O, Haapa K, Heinonen OP, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987. 317:1237–1245.
3. Rubins HB, Robins SJ, Collins D, et al. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999. 341:410–418.
4. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000. 102:21–27.
5. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005. 366:1849–1861.
6. ACCORD Study Group. Ginsberg HN, Elam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010. 362:1563–1574.
7. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999. 340:115–126.
8. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003. 107:363–369.
9. Staels B, Koenig W, Habib A, et al. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature. 1998. 393:790–793.
10. Malik J, Melenovsky V, Wichterle D, et al. Both fenofibrate and atorvastatin improve vascular reactivity in combined hyperlipidaemia (fenofibrate versus atorvastatin trial--FAT). Cardiovasc Res. 2001. 52:290–298.
11. Wu TJ, Ou HY, Chou CW, Hsiao SH, Lin CY, Kao PC. Decrease in inflammatory cardiovascular risk markers in hyperlipidemic diabetic patients treated with fenofibrate. Ann Clin Lab Sci. 2007. 37:158–166.
12. Cortellaro M, Cofrancesco E, Boschetti C, et al. Effects of fluvastatin and bezafibrate combination on plasma fibrinogen, t-plasminogen activator inhibitor and C reactive protein levels in coronary artery disease patients with mixed hyperlipidaemia (FACT study). Fluvastatin Alone and in Combination Treatment. Thromb Haemost. 2000. 83:549–553.
13. Gómez-Gerique JA, Ros E, Oliván J, et al. Effect of atorvastatin and bezafibrate on plasma levels of C-reactive protein in combined (mixed) hyperlipidemia. Atherosclerosis. 2002. 162:245–251.
14. Wang TD, Chen WJ, Lin JW, Cheng CC, Chen MF, Lee YT. Efficacy of fenofibrate and simvastatin on endothelial function and inflammatory markers in patients with combined hyperlipidemia: relations with baseline lipid profiles. Atherosclerosis. 2003. 170:315–323.
15. Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagné C, Couture P. Differential effect of atorvastatin and fenofibrate on plasma oxidized low-density lipoprotein, inflammation markers, and cell adhesion molecules in patients with type 2 diabetes mellitus. Metabolism. 2008. 57:380–386.
16. Muhlestein JB, May HT, Jensen JR, et al. The reduction of inflammatory biomarkers by statin, fibrate, and combination therapy among diabetic patients with mixed dyslipidemia: the DIACOR (Diabetes and Combined Lipid Therapy Regimen) study. J Am Coll Cardiol. 2006. 48:396–401.
17. Jonkers IJ, Mohrschladt MF, Westendorp RG, van der Laarse A, Smelt AH. Severe hypertriglyceridemia with insulin resistance is associated with systemic inflammation: reversal with bezafibrate therapy in a randomized controlled trial. Am J Med. 2002. 112:275–280.
18. Farnier M, Freeman MW, Macdonell G, et al. Efficacy and safety of the coadministration of ezetimibe with fenofibrate in patients with mixed hyperlipidaemia. Eur Heart J. 2005. 26:897–905.
19. Belfort R, Berria R, Cornell J, Cusi K. Fenofibrate reduces systemic inflammation markers independent of its effects on lipid and glucose metabolism in patients with the metabolic syndrome. J Clin Endocrinol Metab. 2010. 95:829–836.
20. Ye P, Li JJ, Su G, Zhang C. Effects of fenofibrate on inflammatory cytokines and blood pressure in patients with hypertriglyceridemia. Clin Chim Acta. 2005. 356:229–232.
21. Okopień B, Krysiak R, Kowalski J, et al. Monocyte release of tumor necrosis factor-alpha and interleukin-1beta in primary type IIa and IIb dyslipidemic patients treated with statins or fibrates. J Cardiovasc Pharmacol. 2005. 46:377–386.
22. Okopień B, Krysiak R, Herman ZS. Effects of short-term fenofibrate treatment on circulating markers of inflammation and hemostasis in patients with impaired glucose tolerance. J Clin Endocrinol Metab. 2006. 91:1770–1778.
23. Kim CJ. Effects of fenofibrate on C-reactive protein levels in hypertriglyceridemic patients. J Cardiovasc Pharmacol. 2006. 47:758–763.
24. Haim M, Benderly M, Tanne D, et al. C-reactive protein, bezafibrate, and recurrent coronary events in patients with chronic coronary heart disease. Am Heart J. 2007. 154:1095–1101.
25. Hiukka A, Westerbacka J, Leinonen ES, et al. Long-term effects of fenofibrate on carotid intima-media thickness and augmentation index in subjects with type 2 diabetes mellitus. J Am Coll Cardiol. 2008. 52:2190–2197.
26. Shin HS, Sung KC, Kim BJ, et al. Effect of exercise on serum C-reactive protein. Korean Circ J. 2005. 35:533–538.
27. Kim SJ, Lee KE, Lee SH, et al. Effect of fibrate on lipoprotein(a) level in hypertriglyceridemic patients. Korean Circ J. 2005. 35:30–36.
28. Ridker PM, Rifai N, Pfeffer MA, et al. Cholesterol and Recurrent Events (CARE) Investigators. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998. 98:839–844.
29. Shen J, Ordovas JM. Impact of genetic and environmental factors on hsCRP concentrations and response to therapeutic agents. Clin Chem. 2009. 55:256–264.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download