Abstract
Mycoplasma pneumoniae (M. pneumoniae) primarily causes respiratory tract infections in persons aged 5-20 years. Tracheobronchitis and bronchopneumonia are the most commonly recognized clinical symptoms associated with M. pneumoniae infection. Complications of this infection are unusual; in particular, cardiac involvement is very rare and is generally accompanied by pneumonia. Nonrespiratory illness can therefore involve direct invasion by M. pneumoniae or autoimmune mechanisms, as suggested by the frequency of cross reaction between human antigens and M. pneumoniae. Herein, we report a case of severe acute myopericarditis with pneumonia caused by M. pneumoniae in a healthy young child who presented with fever, lethargy, oliguria and dyspnea. She survived with aggressive therapy including clarithromycin, intravenous immunoglobulin, inotropics, and diuretics. The patient was discharged on the 19th day after admission and followed up 1 month thereafter at the outpatient clinic without sequelae.
Mycoplasma pneumoniae (M. pneumoniae) is the only recognized human pathogen that is the main cause of respiratory infections in school-aged children and young adults. M. pneumoniae infections occur worldwide and throughout the year; the infection is endemic in large communities, with epidemic outbreaks occurring every 4-7 years. In small communities, the infections are sporadic, with long-lasting and smoldering outbreaks occurring at irregular intervals.1) Community outbreaks largely spread through contact in schools; however, up to 40% of household members of the infected student also develop Mycoplasma infection.2)
M. pneumoniae is a common pathogen that causes upper and lower respiratory tract infections, manifesting as pharyngitis, bronchitis, and atypical pneumonia in children and adolescents.3)
Patients with or without respiratory symptoms can manifest illness involving the skin, central nervous system (CNS), blood, heart, gastrointestinal tract, and joints. Skin lesions include a variety of exanthemas, most notably maculopapular rashes, erythema multiforme, and Stevens-Johnson syndrome.4) Cardiac involvement, such as acute myocarditis, pericarditis, or myopericarditis, is a rare manifestation of M. pneumoniae infection.5) In 1979, Pönkä6) reported that among 560 patients with serologically confirmed M. pneumoniae infection, 25 (4.5%) had carditis (19 perimyocarditis and 6 pericarditis). In contrast, in Korea, only 4 cases of M. pneumoniae-associated pericarditis have been reported in pediatric patients.7-10) Clarithromycin and azithromycin are among the drugs of choice for the treatment of respiratory tract infections. It is important to investigate the association between antibiotic use and resistance as the resistance of common respiratory pathogens to macrolides has been increasing.4) In this report, we present a case of severe acute myopericarditis with pneumonia caused by M. pneumoniae in a healthy 6-year-old girl.
A previously healthy 6-year-old girl was transferred from a primary clinic due to fever, cough, vomiting and lethargy. She was treated with oral azithromycin in a private pediatric clinic for five days. Her past medical history was unremarkable. On the third hospital day, the patient developed lethargy and the fever increased up to 39℃. There was no chest pain, but oliguria and dyspnea were presented. Blood test results showed hemoglobin levels at 12.5 g/dL; white blood cell count was 6700/µL; platelets count was 295000/µL; aspartate aminotransferase was 932 IU/L; alanine transaminase was 449 IU/L; creatinine kinase (CK) was 17122 IU/L; CK-MB was 307 ng/mL; pro-brain natriuretic peptide (pro-BNP) was 16338 pg/mL; lactate dehydrogenase levels were 2857 IU/L; erythrocyte sedimentation rate was 15 mm/h; C-reactive protein was 16 mg/L; and M. pneumoniae IgM Ab was 146.4 U/mL. Abdominal hepatobiliary ultrasound findings suggested ascite and acute hepatitis. Chest radiograph showed signs of bronchopneumonia, mild parapneumonic effusion, and prominent cardiomegaly (Fig. 1A). Echocardiogram showed mild pericardial effusion, prominently decreased left ventricle contractility, enlarged left atrium and ventricle, along with severe mitral regurgitation (Fig. 2A and B). In particular, the left ventricular internal dimension increased 4.41 cm, while the ejection fraction decreased 44.89% (Fig. 3A). On the basis of these findings, acute myocarditis and pericarditis with pericardial effusion were diagnosed. Previously, the patient was administered azithromycin 10 mg/kg/day, a novel macrolide, for five days while in the private pediatric clinic; however, there was no response and the symptoms were aggravated. Therefore, we administered clarithromycin 15 mg/kg/day on the second hospital day, with intravenous ceftriaxone (60 mg/kg/day), and treated the patient with intravenous immunoglobulin (500 mg/kg/day for 5 days) and diuretics, inotropics such as furosemide, spironolactone 2 mg/kg/day on the second hospital day, and dopamine 5 mg/kg/min. On the tenth hospital day, M. pneumoniae IgM Ab increased to 464.2 U/mL in a follow-up laboratory test, and the results of the polymerase chain reaction (PCR) test of the M. pneumoniae-containing sputum were positive. Thereafter, her symptoms gradually improved and appetite was gradually restored. On the thirteenth hospital day, cardiomegaly signs improved on chest radiograph (Fig. 1B). We also observed improvement in the symptoms of upper respiratory tract infection. Furthermore, pericardial effusion had disappeared (Fig. 2C), left ventricle and left atrial dilatation was improved, and left ventricular contractility was normalized (Fig. 3) on follow-up echocardiogram before discharge. On the twentieth hospital day, the patient was discharged without sequela. On follow-up at the outpatient pediatric department, CK-MB and pro-BNP had improved to 25 ng/mL and 107 pg/mL, respectively (Table 1).
The myopericarditis is primarily pericarditic syndrome with minor myocardial involvement, which encompasses the majority of combined pericarditis and myocarditis cases encountered in clinical practice.8) Cardiac complications associated with M. pneumoniae infection are relatively uncommon. The incidence of cardiac involvement ranged from 1% to 8.5% in persons with serological evidence of infection, and it is more common in adults than in children.9) A smaller study with 20 pediatric patients reported eight with purulent pericarditis, six with collagen vascular disease, presumed viral or idiopathic in four, and two with neoplastic mediastinal masses.10) Acute pericarditis is often accompanied by some degree of myocarditis. In clinical practice, both pericarditis and myocarditis co-exist because they share common etiologic agents, which are primarily cardiotropic viruses. Well known causes of myopericarditis are viruses including enterovirus, Epstein-Barr virus, cytomegalovirus, human herpes virus-6, adenovirus, influenza A and B, parvovirus B19, hepatitis B and C, human immunodeficiency virus, and varicella.11)
The causative organism, eventually discovered to be M. pneumoniae, was first isolated in tissue culture from the sputum of a patient with primary atypical pneumonia by Eaton in 1944, and thereafter it was known as the Eaton agent. M. pneumoniae infection is common in children and adolescents, and is often accompanied by extrapulmonary complications. The extrapulmonary complications involving all the main organ systems can occur in association with M. pneumoniae infection as a result of direct invasion and/or autoimmune response.12) Extrapulmonary complications of M. pneumoniae, such as CNS manifestations and arthritis, appear to occur more frequently in children.2) However, the mean age of the patients with M. pneumoniae-associated carditis appears to be relatively greater. Therefore, M. pneumoniae-associated carditis is rare and more commonly described in adults than in children.5)6) Hawkins et al.12) reported that constrictive pericarditis secondary to infection with M. pneumoniae.
The following hypotheses have been proposed for M. pneumoniae-associated carditis: direct invasion of the myocardium by the organism via either the lymphatic or circulatory systems or from the lower respiratory tract by contamination, autoimmune mechanism, or increased tendency for intravascular coagulation.5)10) In general, only 7-11% of the infected patients develop pneumonia, while 5-20% may develop pleural effusions.13) However, Paz and Potasman5) found that 43% of patients with carditis had pneumonia and 19% had pleural effusions. These rather high values raise the question of whether the more severe respiratory cases are indeed associated with the development of carditis. Tachycardia was found in only 43% and chest pain in 38% of the patients studied. Contrary to the abovementioned findings, electrocardiographic (ECG) abnormalities were found in 100% of the reported ECGs. Typical ECG findings of pericarditis were found in 75% of patients. A large study of adult patients reported effusive-constrictive pericarditis in 15 of 190 patients who underwent pericardiocentesis and cardiac catheterization.14)
The best method for identifying pathogenic agents associated with myopericarditis is to obtain tissue directly from the involved area or a pericardial effusion for use in microbiologic cultures.5)11) However, there are significant risks associated with obtaining a sample of tissue from the pericardium. Additional methods for diagnosing M. pneumoniae infection have been developed, including serological methods such as the compliment fixation test, cold agglutination test, particle agglutination test, immunofluorescence antibodies, enzyme immunoassay (EIA) and enzyme-linked immunosorbent assay, as well as PCR-based methods.1) According to Kim et al.15) a 4-fold or more increase in the IgG antibody titer, or a single titer ≥1 : 640 confirms the diagnosis of M. pneumoniae infection. However, when the PCR with Southern hybridization result was combined with the IgM-capture test result with the acute-phase sera, the sensitivity of rapid laboratory diagnosis increased to 95%. So, Waris et al.16) explained that M. pneumoniae IgM serology test is the single most valuable tool for the diagnosis of M. pneumoniae infection in children of any age. At our hospital, the EIA test is performed when M. pneumoniae infection is suspected; if the result of this test showed >70 U/mL IgM titer and/or PCR (+) for specific deoxyribonucleic acid in nasopharyngeal aspirates, the diagnosis of M. pneumoniae infection is confirmed. Diagnosis may be diagnosed through the visualization of echogenic intrapericardial fibrinous strands during echocardiography.17) Echocardiogram is also helpful in documenting the physiologic consequences of constrictive pericarditis, including decreased early diastolic mitral valve inflow velocity with a short deceleration time and reciprocal respiratory variation of left and right ventricular inflow.18)
Clarithromycin (6-O-methylerythromycin) is synthesized by substituting a methoxy group of erythromycin with C-6 hydroxyl group, which produces an acid-stable antimicrobial compound that prevents the degradation of the erythromycin base to the hemiketal intermediate. The increased acid stability of clarithromycin results in improved oral bioavailability and reduced gastrointestinal intolerance. Azithromycin (9-deoxo-9a-aza-9a-methyl-9a-homoerythromycin) is formed by inserting a methyl-substituted nitrogen in place of the carbonyl group at the 9a position of the aglycone ring. The resulting dibasic 15-membered ring macrolide derivative is more appropriately referred to as an "azalide". This structural change increases the acid stability of the compound, significantly increases its serum half-life and tissue penetration, to ultimately result in increased activity against gram-negative organisms and decreased activity against some gram-positive organisms when compared with erythromycin.19) Both azithromycin and clarithromycin achieve high extracellular concentrations in respiratory tissue and are commonly used as a first-line empirical treatment for community-acquired respiratory tract infections. However, they differ substantially in their plasma half-life and tissue persistence, which are both very important factors in the selection of macrolide-resistant organisms.13)19) For instance, the time required for the clearance of a drug or reduction in its concentration to below the minimum inhibitory concentration is between 5 and 7 half-lives. Therefore, since the plasma half-life of azithromycin is 68 hours, it may persist in vivo for at least 3-4 weeks after treatment. In contrast, clarithromycin has a half-life of only 5-7 hours, and therefore it exerts little post-treatment effect. This difference explains why azithromycin use is selected for significantly more macrolide-resistant streptococci until about 4 weeks than compared with clarithromycin.20)
In conclusion, we report the case of child who was successfully treated with oral clarithromycin, intravenous ceftriaxone, intravenous immunoglobulin and other cardiac supportive medications after confirming M. pneumoniae infection-associated acute myopericarditis with symptoms of upper respiratory infection. M. pneumoniae should be considered in the differential diagnosis of pathogens in acute myopericarditis patients. It is important to administer appropriate treatment to achieve a good outcome for Mycoplasma-associated carditis, and furthermore, serological testing for Mycoplasma species should be included in the routine workup of carditis of unknown cause.
Figures and Tables
Fig. 1
Pretreatment and post-treatment chest radiographic findings. A: bronchopneumonia, scanty parapneumonic effusion, and cardiomegaly (CT ratio, 0.66) was observed (HD#3). B: there was no abnormal finding after treatment, including heart size (HD#13). HD: hospital day.
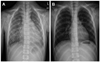
Fig. 2
Pretreatment echocardiogram in apical 4-chamber view findings. A: pericardial effusion (arrow) was observed (HD#3). B: severe mitral regurgitation was observed (HD#3). C: there was no abnormal finding after treatment, including pericardial effusion (HD#16). HD: hospital day.

Fig. 3
Pretreatment (A: HD#3) and post-treatment (B: HD#16) echocardiogram in apical M-mode findings. HD: hospital day.
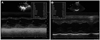
Table 1
Laboratory data

*Virus study: influenza virus A, B, respiratory syncytial virus, rhinovirus A, adenovirus, parainfluenza virus, metapneumovirus. Hb: hemoglobin, WBC: white blood cell, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactic dehydrogenase, CRP: C-reactive protein, BNP: B type natriuretic peptide, PCR: polymerase chain reaction, CK-MB: creatine kinase-MB, M. pneumoniae: Mycoplasma pneumoniae
Acknowledgments
This work was supported by a research grant funded by the Wonkwang University in 2011.
References
1. Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008. 32:956–973.
2. Defilippi A, Silvestri M, Tacchella A, et al. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Respir Med. 2008. 102:1762–1768.
3. Waites KB. New concepts of Mycoplasma pneumoniae infections in children. Pediatr Pulmonol. 2003. 36:267–278.
4. Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004. 113:701–707.
5. Paz A, Potasman I. Mycoplasma-associated carditis: case reports and review. Cardiology. 2002. 97:83–88.
6. Pönkä A. Carditis associated with mycoplasma pneumoniae infection. Acta Med Scand. 1979. 206:77–86.
7. Kim DI, Choi JH, Cho EY, et al. A case of fatal myocarditis associated with Mycoplasma pneumoniae pneumonia. Korean J Pediatr Infect Dis. 2009. 16:92–96.
8. Imazio M, Cecchi E, Demichelis B, et al. Myopericarditis versus viral or idiopathic acute pericarditis. Heart. 2008. 94:498–501.
9. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004. 17:697–728.
10. Roodpeyma S, Sadeghian N. Acute pericarditis in childhood: a 10-year experience. Pediatr Cardiol. 2000. 21:363–367.
11. Imazio M, Trinchero R. Myopericarditis: etiology, management, and prognosis. Int J Cardiol. 2008. 127:17–26.
12. Hawkins S, Rausch CM, McCanta AC. Constrictive pericarditis secondary to infection with Mycoplasma pneumoniae. Curr Opin Pediatr. 2011. 23:126–129.
13. Mansel JK, Rosenow EC 3rd, Smith TF, Martin JW Jr. Mycoplasma pneumoniae pneumonia. Chest. 1989. 95:639–646.
14. Sagristà-Sauleda J, Angel J, Sánchez A, Permanyer-Miralda G, Soler-Soler J. Effusive-constrictive pericarditis. N Engl J Med. 2004. 350:469–475.
15. Kim NH, Lee JA, Eun BW, et al. Comparison of polymerase chain reaction and the indirect particle agglutination antibody test for the diagnosis of Mycoplasma pneumoniae pneumonia in children during two outbreaks. Pediatr Infect Dis J. 2007. 26:897–903.
16. Waris ME, Toikka P, Saarinen T, et al. Diagnosis of Mycoplasma pneumoniae pneumonia in children. J Clin Microbiol. 1998. 36:3155–3159.
17. Kim SH, Song JM, Jung IH, Kim MJ, Kang DH, Song JK. Initial echocardiographic characteristics of pericardial effusion determine the pericardial complications. Int J Cardiol. 2009. 136:151–155.
18. Asher CR, Klein AL. Diastolic heart failure: restrictive cardiomyopathy, constrictive pericarditis, and cardiac tamponade: clinical and echocardiographic evaluation. Cardiol Rev. 2002. 10:218–229.
19. Piscitelli SC, Danziger LH, Rodvold KA. Clarithromycin and azithromycin: new macrolide antibiotics. Clin Pharm. 1992. 11:137–152.
20. Malhotra-Kumar S, Lammens C, Coenen S, Van Herck K, Goossens H. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet. 2007. 369:482–490.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download