Abstract
Herein, we present a case of a successful catheter ablation of ventricular tachycardia (VT) using a radial artery approach in a post-myocardial infarction patient, who had an implantable cardioverter-defibrillator and peripheral artery disease. Although the patient did not use antiarrhythmic drugs, the patient experienced no recurrence of VT during the following 3-year period.
Peripheral artery disease (PAD) is comorbid in 10.6% of patients with ischemic heart disease.1) The difficulty in accessing the arterial system may complicate ventricular tachycardia (VT) ablation procedures in patients with PAD. The radial artery is the preferred access route for patients undergoing percutaneous coronary intervention, in terms of the patients' comfort and in terms of a decreased risk for vascular complications.2)3) Herein, we present a case of a successful catheter ablation of VT by radial artery access in a post-myocardial infarction patient with PAD, who had previously failed ablation via a transseptal approach.
A 73 year-old man with prior anterior myocardial infarction was admitted for management of an electrical storm (ES) occurring 20 months after the implantation of an implantable cardioverter-defibrillator (ICD). His medical conditions included an anterior myocardial infarction that had occurred 15 years prior, heart failure, peripheral arterial disease, non-disabling stroke, hypertension, gout, and diabetes mellitus. He underwent implantation of dual chamber ICDs due to sustained VT (Fig. 1A and B). At the time, the left ventricular (LV) ejection fraction was 36%, and a LV aneurysm was noted. Coronary angiography showed a 50% stenosis in the mid-portion of the left anterior descending artery, 80% stenosis at the distal left circumflex artery, and 40% stenosis in the proximal right coronary artery. His medications included a beta-blocker, amiodarone, an angiotensin-converting enzyme inhibitor, aspirin, and diuretics. Computed tomography angiography revealed extensive calcification from the distal abdominal aorta to the bilateral common iliac artery and the tortuous right common iliac artery (Fig. 1C and D).
The patient underwent catheter ablation for medically refractory, frequent premature ventricular complexes (60% of total beats) that were originating from the left apico-inferior septum. The ablation was performed by a transseptal approach due to the deterioration of heart failure, which had begun 13 months before presentation (Fig. 2).
Before the occurrence of the ES, there was only one episode of VT treated with anti-tachycardia pacing. Sixteen episodes of VT with a cycle length of 430 to 455 ms occurred per day, and 3 episodes were terminated by cardioversion (20 J).
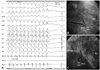
After obtaining informed consent from the patient, he underwent catheter ablation of VT via a transseptal approach, using an external irrigation catheter (power-controlled mode, 30 Watts, 45℃, 17 mL/min). During the procedure, VT was still inducible by programmed electrical stimulation after ablation, guided by entrainment mapping and pacemapping.4) Due to limitations in catheter localization in LV anterior and lateral wall, VT was still inducible after the ablation. Procedural and radiofrequency delivery times were 6 hours and 30 minutes for this procedure, respectively. The patient underwent a second catheter ablation of VT via the left radial artery 4 days later. Access to the radial artery was performed after confirmating normal results of an Allen's test. During a 2nd session, a 6 Fr ablation catheter with a 4 mm distal tip was used for mapping and ablation (temperature-controlled mode, 50 Watts, 60℃). In brief, we induced VT by extra stimulation up to S4 from the right ventricular apex or outflow tract with/without isoproterenol infusion. When VT was induced, ablation catheter was moved systematically around the LV from apex to the mid-ventricular level, inferior to superior and septal to lateral to search for presystolic and diastolic potentials. In hemodynamically stable VT, entrainment mapping was performed and delivery of RF energy was done. If VT was terminated in less than 20 seconds, RF energy was delivered for 60 seconds and booster energies were delivered around the site. If VT was not terminated in less than 20 seconds, delivery of RF energy was stopped. During sinus rhythm, we mapped the late potential in the apex and border zone of aneurysm and normal myocardium. When late potential was found, we performed pacemapping and delivery RF energy where 11-12/12 leads of pacemap was matched to induced VT. After applications of RF energy, we repeated VT induction and delivery of RF energy until stable VT was not induced. A total of 17 sustained VT forms were induced, and 10 forms of VT were successfully terminated with radiofrequency energy during the 1st and 2nd VT ablations (Fig. 3) (Supplementary data). Major sites of ablation were LV anterior, anterolateral, inferolateral, inferior wall between LV aneurysm and normal LV myocardium. Three forms of VT were treated with guidance from pace mapping. After the ablation procedure, only hemodynamically unstable VT was induced by triple ventricular extra stimulation and externally cardioverted. Procedural and radiofrequency delivery times were 3 hours and 36 minutes for the 2nd procedure, respectively. The procedure was completed without complications. After the VT ablation procedure, the patient had no recurrence of premature ventricular complex (PVC) or VT during a 3-year follow-up period, without the use of amiodarone.
We have presented a successful catheter ablation of a VT ES, via the radial artery, in a patient with remote myocardial infarction and PAD. This is the first report on the use of the radial artery in ablation of VT in Korea.
Frequent PVC can cause reversible ventricular dysfunction in patients both with and without structural heart disease.5)6) Purkinje fibers are resistant to ischemic insult,7) and surviving Purkinje fibers may participate in reentrant circuits of VT.8)9) In addition, Purkinje fiber-related VPC can precipitate polymorphic VT and ventricular fibrillation in susceptible patients.10)11) Mapping and selectively ablating PVC, guided by Purkinje potential, is effective in some patients with remote myocardial infarction.12)
Electrical storms occur in 10-23% of patients with implantable cadioverter-defibrillators.13-15) Prophylactic treatment with amiodarone and beta-blockers decreases the occurrence of VT.16) Catheter ablation is needed to control ES in patients with structural heart disease that is refractory to antiarrhythmic drugs.10)17)
In this case, a 2nd catheter ablation procedure was needed to control the ES. Access to the LV is usually achieved via a retrograde aortic approach. However, in patients with extensive aortic and peripheral arterial disease, access through the femoral artery carries the potential risk of cholesterol embolism and stroke; a transseptal approach to the LV circumvents this risk. Another option in patients with extensive peripheral arterial disease is access via the brachial or radial arteries.18) Because of easy maneuverability of catheter movement in the septum, as well as the anterior and lateral left ventricle from the radial approach, multiple forms of VT were successfully ablated in the second session. Though we were not limited in our ability to manipulate the ablation catheter via the radial artery, access through the brachial artery may carry a lower risk of arterial spasm.
There are several limitations to this study. First, prophylactic catheter ablation before the implantation of a defibrillator decreases the occurrence of ventricular tachyarrhythmia.19) Earlier catheter ablation may have prevented late ESs in this case. Second, arterial access using a long sheath, together with use of an electro-anatomic mapping system, may be sufficient for preventing peripheral complications, assisting with the delineation of anatomic circuits for multiple VTs and decreasing procedural time. Third, catheter ablation using a Stereotaxis Niobe system may facilitate mapping of the left ventricle via a transseptal approach.
In conclusion, catheter ablation of VT storms can be performed using a transradial approach to the left ventricle and a conventional electrophysiologic mapping maneuver in patients with prior myocardial infarction and peripheral vascular disease. The successful ablation of VT made the use of antiarrhythmic drugs unnecessary in this case.
Figures and Tables
Fig. 1
Electrocardiograms and angiograms of the patient. A: a 12-lead electrocardiogram of initial ventricular tachycardia had configuration of a left bundle branch block and left axis deviation. B: a 12-lead electrocardiogram after restoration of sinus rhythm. C: extensive calcification of the abdominal aorta and iliac arteries were noted in computed tomography angiography. D: a very tortuous, angulated right common iliac artery is shown.
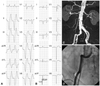
Fig. 2
Intracardiac tracings (A) and right and left anterior oblique fluoroscopic image (B and C) mapping of the premature ventricular complex. The surface electrocardiogram, proximal and distal bipolar electrograms from the ablation catheter (Abl p, Abl d), His recording catheter (His p, His d), right ventricular apex (RVA), and arterial pressure are shown from top to bottom. A Purkinje-like potential, preceding QRS onset of PVC by 112 ms, is found in the left ventricular apico-inferior septum. The application of radiofrequency energy at the site abolished PVC in seconds. The A-lead and ICD lead denote atrial and ICD leads, respectively. PVC: premature ventricular complex, ICD: implantable cardioverter-defibrillator.
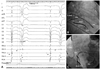
Fig. 3
Intracardiac tracings (A) and right and left anterior oblique fluoroscopic images (B and C) of ventricular tachycardia ablation approached from the left radial artery. Surface electrocardiograms, proximal and distal bipolar electrograms from the ablation catheter (Abl p, Abl d) and right ventricular apex (RVA) are displayed from top to bottom. Mid-diastolic potential was recorded prior to the onset of VT by 114 ms and the application of radiofrequency energy terminated the VT within seconds (A). The successful ablation sites (anterolateral left ventricular apex) are shown in B and C. The A-lead and ICD lead denote atrial and ICD leads, respectively. VT: ventricular tachycardia, ICD: implantable cardioverter-defibrillator.
Acknowledgments
This work was supported by a 2012 research grant from Pusan National University Yangsan Hospital.
References
1. Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006. 295:180–189.
2. Kim JY, Yoon J, Jung HS, et al. Feasibility of the radial artery as a vascular access route in performing primary percutaneous coronary intervention. Yonsei Med J. 2005. 46:503–510.
3. Choussat R, Black A, Bossi I, Fajadet J, Marco J. Vascular complications and clinical outcome after coronary angioplasty with platelet IIb/IIIa receptor blockade: comparison of transradial vs transfemoral arterial access. Eur Heart J. 2000. 21:662–667.
4. Stevenson WG, Friedman PL, Sager PT, et al. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol. 1997. 29:1180–1189.
5. Takemoto M, Yoshimura H, Ohba Y, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005. 45:1259–1265.
6. Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm. 2009. 6:1543–1549.
7. Friedman PL, Stewart JR, Fenoglio JJ Jr, Wit AL. Survival of subendocardial Purkinje fibers after extensive myocardial infarction in dogs. Circ Res. 1973. 33:597–611.
8. Bogun F, Good E, Reich S, et al. Role of Purkinje fibers in post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006. 48:2500–2507.
9. Bogun F, Crawford T, Chalfoun N, et al. Relationship of frequent postinfarction premature ventricular complexes to the reentry circuit of scar-related ventricular tachycardia. Heart Rhythm. 2008. 5:367–374.
10. Bänsch D, Oyang F, Antz M, et al. Successful catheter ablation of electrical storm after myocardial infarction. Circulation. 2003. 108:3011–3016.
11. Szumowski L, Sanders P, Walczak F, et al. Mapping and ablation of polymorphic ventricular tachycardia after myocardial infarction. J Am Coll Cardiol. 2004. 44:1700–1706.
12. Sarrazin JF, Good E, Kuhne M, et al. Mapping and ablation of frequent post-infarction premature ventricular complexes. J Cardiovasc Electrophysiol. 2010. 21:1002–1008.
13. Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH. Electrical storm in patients with transvenous implantable cardioverter-defibrillators: incidence, management and prognostic implications. J Am Coll Cardiol. 1998. 32:1909–1915.
14. Exner DV, Pinski SL, Wyse DG, et al. Electrical storm presages nonsudden death: the antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation. 2001. 103:2066–2071.
15. Hohnloser SH, Al-Khalidi HR, Pratt CM, et al. Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. Eur Heart J. 2006. 27:3027–3032.
16. Connolly SJ, Dorian P, Roberts RS, et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA. 2006. 295:165–171.
17. Schreieck J, Zrenner B, Deisenhofer I, Schmitt C. Rescue ablation of electrical storm in patients with ischemic cardiomyopathy: a potential-guided ablation approach by modifying substrate of intractable, unmappable ventricular tachycardias. Heart Rhythm. 2005. 2:10–14.
18. Lee DW, Kim J, Lee HC, et al. Catheter ablation of a left free-wall accessory pathway via the radial artery approach. Yonsei Med J. 2007. 48:1048–1051.
19. Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007. 357:2657–2665.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download