Abstract
Aorto-right ventricular fistula is a very rare complication of aortic dissection. We report a case of acute aortic dissection extending into the right ventricle as documented by echocardiography. The patient survived after successful surgical repair.
Acute aortic dissection is an uncommon complication in patients with chronic hypertension. The separation of the layers within the aortic wall results in a false lumen with penetration of blood into the intima-media space. Many complications may ensue as the false channel enlarges, including myocardial infarction, acute heart failure, and rupture to neighboring structures.1) We report a patient who underwent successful surgical repair of an aortic dissection that had caused an aorta-right ventricular fistula.
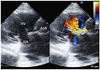
A 66-year-old woman presented to the emergency department of our center with complaints of melena and back pain in April 2010. The patient had a medical history that included hypertension which had been treated for 15 years with medication, an aortic valve replacement, and a MAZE procedure for degenerative aortic valve regurgitation 3 years prior. Her admission systemic blood pressure was 140/110 mm Hg, her pulse rate was 72 bpm, and her respiratory rate was 20 breaths per minute. A continuous murmur was noted at that time. A chest radiograph showed cardiomegaly with cardiothoracic index of 62%. Ventricular premature complex and left ventricular hypertrophy were noted on electrocardiogram. Esophagogastroscopy revealed no abnormal findings. Transthoracic echocardiography showed severely dilated aortic root and ascending aorta with intimal flap suggestive of aortic dissection (Fig. 1). Color Doppler demonstrated a shunt flow from aortic root to right ventricle, probably representing an aorto-right ventricular fistula (Fig. 2). The prosthetic aortic valve was normal. The left and right ventricles had normal function. Without further aortography or cardiac catheterization, we conducted emergency cardiac surgery. In the operation field, we found that the ascending aorta was severely enlarged. Although the prosthetic aortic valve was intact, the organized flap extended to the aortic root. A perivalvular adhesion was seen between the right atrium and the aortic wall, and a fistula had formed towards the right ventricle with less adhesion (Fig. 3). We conducted a modified Bentall operation with closure of the fistula. At 2 weeks post-surgery, echocardiography showed insignificant shunt flow from the ascending aorta to the right ventricle. The patient's condition improved and she was discharged 20 days after surgery.
Acute aortic dissection is a life-threatening disease and may cause serious complications.2) Penetration into a cardiac chamber producing an aorto-cameral fistula is a very rare such complication. In 1924, Boyd3) reviewed 4000 autopsy reports of thoracic aortic aneurysm and found 1197 instances of rupture. The authors found several cases of fistula surrounding structures but no aortoright ventricular fistula. Although aortocameral fistulas have been rarely reported, the incidence tends to increase with previous cardiac surgery, particularly aortic valve replacement for aortic regurgitation.4) When aortic dissections occur in patients with previous cardiac surgery, postoperative adhesion may prevent free rupture into the pericardial space however these tend to penetrate into a neighboring cardiac chamber.5)6)
To diagnose a fistula into a neighboring structure found on aortic dissection, clinical history, physical examination, echocardiography, aortography, CT, and MRI may be helpful. Although aortography is the gold standard for diagnosis, non-invasive methods such as contrast enhanced CT, MRI, and echocardiography are currently preferred. Transesophageal echocardiography has better sensitivity and specificity than transthoracic echocardiography.7)8) Mortality in type A dissection after surgery is 26%, increasing up to 58% in cases that did not undergo surgery.9) Therefore, early diagnosis and immediate surgical intervention are important to reduce mortality in patients with aortic dissection. In this respect, transesophageal echocardiography is very helpful in aortic dissection enabling both diagnosis and detection of fistula by aortic dissection. CT, MRI, and aortography may be needed to confirm the diagnosis of aorto-cameral fistula, especially if echocardiography provides insufficient information. Although fistulization (aorto-right atrial and aorto-pulmonary) after acute dissection has been well documented, there are few reports of a fistula opening into the right ventricle.10-14)
Although aortic dissection complicated by a fistula is extremely rare, it should be suspected in any patient with a history of previous cardiac surgery, dyspnea, or murmur. In particular, echocardiography with Doppler is greatly helpful in checking the shunt caused by the fistula, as well as in visualization of the intimal flap.
Figures and Tables
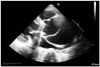
Fig. 1
Transthoracic echocardiography showed severely increased aortic root and ascending aorta with intimal flap.
References
1. Spier LN, Hall MH, Nelson RL, Parnell VA, Pogo GJ, Tortolani AJ. Aortic dissection: rupture into right ventricle and right pulmonary artery. Ann Thorac Surg. 1995. 59:1017–1019.
2. Jo YJ, Lee EJ, Oh JW, et al. Aortic dissection and rupture in a child. Korean Circ J. 2011. 41:156–159.
3. Boyd LJ. A study of four thousand reported cases of aneurysm of the thoracic aorta. Am J Med Sci. 1924. 168:654–663.
4. Lindsay J Jr. Aortocameral fistula: a rare complication of aortic dissection. Am Heart J. 1993. 126:441–443.
5. Estrera AL, Miller CC, Kaneko T, et al. Outcomes of acute type a aortic dissection after previous cardiac surgery. Ann Thorac Surg. 2010. 89:1467–1474.
6. Gillinov AM, Lytle BW, Kaplon RJ, Casselman FP, Blackstone EH, Cosgrove DM. Dissection of the ascending aorta after previous cardiac surgery: differences in presentation and management. J Thorac Cardiovasc Surg. 1999. 117:252–260.
7. Caruso A, Iarussi D, Materazzi C, et al. Aortic dissection with fistula to left atrium: diagnosis by transesophageal echocardiography with successful repair. J Am Soc Echocardiogr. 2000. 13:69–72.
8. Berman AD, Come PC, Riley MF, Weintraub RM, Johnson RG, Aroesty JM. Two-dimensional and Doppler echocardiographic diagnosis of an aortic to right atrial fistula complicating aortic dissection. J Am Coll Cardiol. 1987. 9:228–230.
9. Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000. 283:897–903.
10. Alam M. Transesophageal color flow Doppler features of aorta to right ventricle fistula. Chest. 1993. 103:1907–1908.
11. Perryman RA, Gay WA. Rupture of dissecting thoracic aortic aneurysm into the right ventricle. Am J Cardiol. 1972. 30:277–281.
12. Marek D, Nemec P, Maderová K, et al. Acute aortic dissection complicated by fistula from aorta to right ventricle through ventricular septum. Cardiovasc Pathol. 2009. 18:301–307.
13. Davies NJ, Butany J, Yock PG, Rakowski H. Aortic dissection and rupture that produce right ventricular perforation: detection by echocardiography and color flow mapping. J Am Soc Echocardiogr. 1990. 3:140–144.
14. McGoldrick JP, Wells FC. Type 1 aortic dissection with right coronary artery occlusion and fistula to right atrium and right ventricle. Eur J Cardiothorac Surg. 1990. 4:514–516.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download