Introduction
Idiopathic ventricular fibrillation (VF) is defined as VF of undetermined etiology in the absence of structural heart disease and it accounts for 5-10% of survivors of out-of-hospital sudden cardiac arrest.1) Recurrent idiopathic VF triggered by unifocal premature ventricular complexes (PVCs) can be managed by radiofrequency catheter ablation.2-4) We report a case of unifocal PVC ablation for recurrent idiopathic VF.
Case
A 17-year-old man was transferred to Dong-A University Hospital from a regional hospital for resuscitation after sudden cardiac arrest. The patient suddenly collapsed in his classroom. Cardiopulmonary resuscitation (CPR) was performed immediately by classmates. An emergency medical service team arrived at the place of arrest within 5 minutes and an automated external defibrillator (AED) was applied. Documented presenting rhythm was VF, which was successfully terminated by AED shock. The patient was admitted to a regional hospital. Frequent PVCs were detected during electrocardiogram (ECG) monitoring and VF that required subsequent CPR recurred approximately 2 hours after the first episode of cardiac arrest. Defibrillation was performed and the patient regained consciousness without significant hypoxic brain injury10 hours later. The patient was transferred to our hospital for implantable cardioverter-defibrillator (ICD) implantation on the next day. Herein, we report our experience of idiopathic VF ablation.
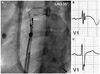
Vital signs were stable and physical examinations were unremarkable. The patient had no past history of palpitations or syncope. Furthermore, there was no family history of syncope or sudden cardiac death. The patient did not take any medication including herbs or supplementary health foods for a minimum of 3 months prior to this cardiac arrest. Serum sodium and potassium concentrations were 137 and 3.8 mmol/L, respectively. Serum magnesium and ionized calcium concentrations were 2.3 and 4.5 mg/dL, respectively. Cardiac troponin-I and brain natriuretic peptide levels were 0.05 ng/mL and 76.2 pg/mL, respectively. Post-resuscitation standard 12-lead ECGs showed sinus rhythm with normal P waves and QRS complexes. Corrected QT interval (340-440 ms) and ST-T wave morphology were normal. Fragmented QRS complex with T wave inversion was noted in V1. There were frequent monomorphic PVCs with a coupling interval of 400 ms. Monomorphic PVCs had left bundle branch block configuration and inferior axis (Fig. 1). The patient was admitted to coronary care unit for ECG monitoring. Isolated monomorphic PVCs with bigeminy and trigeminy were detected frequently. Couplets and triplets were occasionally detected. VF requiring CPR recurred 42 hours after the first episode of cardiac arrest. Recorded rhythm analysis revealed two episodes VFs. In both episodes, fast polymorphic ventricular tachycardia was induced by a triplet of monomorphic PVCs having nearly identical QRS morphology with isolated PVCs (Fig. 1). Induced fast polymorphic ventricular tachycardia degenerated to VF. Idiopathic VF triggered by frequent unifocal PVCs of right ventricular outflow tract (RVOT) origin was suspected and ICD implantation was planned for recurrent VF. Propranolol was given to reduce the burden of PVCs and frequent ICD discharges. However, frequent monomorphic PVCs with occasional couplets and triplets were still detected despite oral administration of propranolol 240 mg/day for 2 days.
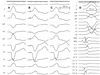
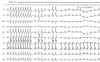
Therefore, we performed emergent radiofrequency catheter ablation for monomorphic PVCs. A 20-pole variable circular mapping catheter (Lasso 2515, Biosense-Webster, Diamond Bar, CA, USA) and a deflectable ablation catheter (EPT, 7 Fr, 4 mm tip, Large curve, Boston Scientific, Natick, MA, USA) were inserted into RVOT using long guiding sheathes (SL-1, Daig Corporation, Minnetonka, MN, USA) by trans-femoral venous approach (Fig. 2). Isolated monomorphic PVCs those triggered VF appeared frequently during electrophysiologic study (Fig. 3A). The earliest ventricular activation signal was recorded at anterolateral wall of RVOT 2 cm below the pulmonary valve during activation mapping for spontaneous PVCs. Radiofrequency catheter ablation was performed where the QRS morphology of paced beat was nearly identical with spontaneous PVCs (Fig. 3B). The earliest ventricular activation signal was recorded 20 ms before the QRS onset in the ablation catheter (Fig. 3D). Temperature and power controls were set to 55℃ and 30 Watts, respectively. Accelerated ventricular rhythm having nearly identical QRS morphology with spontaneous PVCs was induced transiently after starting the first ablation (Fig. 3C). Frequent monomorphic PVCs disappeared during ongoing radiofrequency energy delivery and were not detected anymore after finishing the first ablation for 60 seconds (Fig. 4).
Booster radiofrequency energies were given around the successfully ablated site. Monomorphic PVCs triggering VF were not inducible by programmed electrical stimulation with IV isoproterenol after the final ablation. The ablation procedure was finished without complication. After ablation, the fragmentation of the QRS complex and T wave inversion in V1 disappeared (Fig. 2). Exercise treadmill test (up to the stage 4 by Bruce protocol) and isoproterenol provocation test (up to the dose of 7 µg/min) were performed 2 days after ablation; neither induces any PVCs.
No structural heart disease was revealed by echocardiography, cardiac magnetic resonance imaging focused on RVOT, and coronary angiography with ergonovine provocation. Flecainide and epinephrine provocation tests for Brugada and congenital long QT syndromes did not induce significant ST elevation and QT prolongation. Recurrent idiopathic VF triggered by unifocal PVCs of RVOT origin was confirmed after exclusion of underlying structural heart diseases and ion-channelopathies.
There was no recurrence of VF for the observation period of 2 weeks. On the day after radiofrequency ablation, telemetry monitoring and repeated 24-hour ambulatory ECG monitoring detected only 0 to 4 PVCs with different coupling intervals (440-480 ms) and morphologies. After confirming successful eradiation of unifocal RVOT-PVCs triggering VF, a single chamber ICD was implanted for secondary prevention of sudden cardiac arrest due to VF. The patient was discharged with a prescription for oral bisoprolol 10 mg/day.
Discussion
Successful management of idiopathic VF by radiofrequency catheter ablation for unifocal PVCs triggering VF was previously reported.2-4) In the reports, ablation was performed to prevent recurrent VF or frequent ICD discharges. In this patient, 4 episodes of VF that requires CPR occurred within the initial 42 hours period. Two episodes of VF initiated by monomorphic PVCs were documented. PVCs triggering VF had nearly identical QRS morphology with isolated PVCs suggesting their unifocal origin.
The origin of PVCs triggering VF was speculated as RVOT based on their QRS morphology of standard 12 lead ECG with typical left bundle branch block configuration and inferior axis. In patients with idiopathic VF, RVOT is a relatively uncommon origin of PVCs triggering VF accounting for only 15%.2) Although PVCs or ventricular tachycardia originated from RVOT respond to beta blockers,5) propranolol therapy (240 mg/day for two days) did not reduce the burden of monomorphic PVCs in this patient. Therefore, we performed emergent radiofrequency catheter ablation to prevent recurrent VF and frequent ICD discharges. Fortunately, enough number of PVCs appeared for appropriate activation mapping during electrophysiologic study. The exact locus of the PVC origin was confirmed as anterolateral wall of RVOT 2 cm below pulmonary valve by both activation and paced mappings. The earliest ventricular activation 20 ms earlier to the QRS onset during activation mapping, matched QRS morphology between paced beats and spontaneous PVCs, induction of accelerated ventricular rhythm having nearly identical QRS morphology with spontaneous PVCs suggested appropriate selection of ablation site. After radiofrequency ablation, the daily burden of monomorphic PVCs was reduced less than 0.005% of the daily total beats and VF did not recur. Compared to clinical PVCs triggering VF, rare PVCs after ablation had somewhat different QRS morphologies and slightly prolonged coupling intervals suggesting their different origin or significant substrate modification. No induction of clinical PVCs by exercise treadmill test and isoproterenol provocation test also suggested successful ablation. Disappearance of fragmented QRS complex with T wave inversion in V 1 may be a signal for successful modulation of VF substrate in right ventricle.2) The fragmented QRS complex in V 1 did not reappear during the 2 month follow up period over.
Eradication of unifocal PVCs that triggered recurrent idiopathic VF by radiofrequency catheter ablation is a challenging issue for electrophysiologists. Successful management of unifocal PVCs triggering VF by radiofrequency ablation may provide another treatment option for sudden cardiac arrest survivors. If several events of isolated PVC are present to allow for appropriate activation mapping, ablation therapy should be considered to minimize the risk of recurrent VF and frequent ICD discharges. Technical successfulness of radiofrequency ablation for idiopathic VF initiated by RVOT-PVCs was proven by previous studies.1)2)6)7) Although long term outcome of idiopathic VF ablation is not certain due to limited data, radiofrequency ablation may reduce the burden of triggering PVCs and VF recurrence.6)7) We successfully ablated RVOT-PVCs triggering recurrent VFs in a 17-year-old man without structural heart disease before ICD implantation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download