Abstract
Sarcoidosis is a rare but potentially fatal multisystem granulomatous disease of unknown etiology. While a number of clinical manifestations may develop, cardiac involvement (prior to or coincident with sarcoidosis of other organs) is an important prognostic factor. Recently, we encountered a patient with cardiac sarcoidosis who presented with complete atrioventricular (AV) block and sustained ventricular tachycardia. An implantable cardioverter-defibrillator was inserted as a precautionary measure for ventricular tachycardia and symptomatic complete AV block. 18F-fluoro-2-deoxyglucose positron emission tomography confirmed a dramatic response to high-dose steroid at four weeks, as demonstrated by a marked decrease in cardiac sarcoid activity from baseline status.
Sarcoidosis is a multisystem granulomatous disease of unknown etiology. Cardiac manifestations, seen in approximately 5% of patients,1)2) typically include congestive heart failure with left ventricular dysfunction, conduction abnormalities, and ventricular arrhythmias. Moreover, sudden death has been reported in up to 67% of instances where cardiac sarcoidosis is diagnosed postmortem.3) Herein, we describe a patient whose cardiac sarcoidosis presented as symptomatic complete atrioventricular (AV) block with congestive heart failure and ventricular tachycardia. Tissue diagnosis was achieved via paratracheal node sampling, obtained by endobronchial ultrasonography guided transbronchial lymph node aspiration (EBUS-TBNA). The patient then received an implantable cardioverter-defibrillator (ICD) and systemic steroid therapy. Follow-up 18F-fluoro-2-deoxyglucose positron emission tomography (18F-FDG-PET) showed a marked decrease in cardiac sarcoid activity compared to the baseline.
A 55-year-old male was admitted to another hospital six months previously for dyspnea on exertion (DOE) and dizziness. At that time, there was evidence of severe global hypokinesia of the left ventricle (LV), with systolic dysfunction {left ventricular ejection fraction (LVEF) 33% per M-mode} on echocardiogram, as well as enlargement of the right atrium and right ventricle (RV), and severe RV dysfunction. A corresponding electrocardiogram documented complete right bundle branch block with sinus bradycardia, while 24-hour Holter monitoring recorded a maximum RR interval of 2.76 seconds. No high-level AV block or arrhythmia was apparent, and by coronary angiography, epicardial coronary arteries were normal. On this basis, a diagnosis of idiopathic dilated cardiomyopathy was rendered and thereafter treated with ramipril 2.5 mg, furosemide 20 mg, and spironolactone 25 mg daily. Upon admission to our center, the patient again presented with DOE (NYHA class III) and intermittent dizziness, which were both aggravated in the preceding 15 days. His electrocardiogram showed complete AV block (heart rate, 27 beats per minute) (Fig. 1A) as did a 24-hour Holter monitor, with a maximum RR interval of 4.7 seconds. An echocardiogram demonstrated severe LV dysfunction (LVEF 34.4% per modified Simpson method), a dyskinetic basal septum, basilar cardiac akinesia, and an enlarged, severely dysfunctional RV, without pulmonary hypertension {pulmonary artery systolic pressure (RVSP) 27.64 mm Hg} (Fig. 1B). Mild cardiomegaly was noted on chest X-ray (cardiothoracic ratio 55%), but hilar lymph nodes were not prominent (Fig. 1C). Gadolinium-dietylene triamine penta-acetic acid (DTPA)-enhanced cardiac MRI showed delayed hyper-enhancement of the entire RV wall and subepicardial (apical) or transmural (apical, basal) portions of the LV (Fig. 1D).
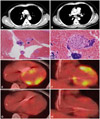
While cardiac sarcoidosis was strongly suspected, endomyocardial biopsy findings were nonspecific, and angiotensin-converting enzyme (ACE) was within normal reference range (27.5 IU/L; normal range: 8.0-55.0 IU/L). During endomyocaridal biopsy, sustained ventricular tachycardia developed repetitively (Fig. 1E). Enlarged right paratracheal (17.27×8.35 mm) and subcarinal (18.63×11.97 mm) lymph nodes, found incidentally with coronary CT angiography six months prior (Fig. 2A and B, respectively), were instead sampled via EBUS-TBNA to histologically confirm the presence of noncaseating epitheloid granulomata with giant cells (Fig. 2C and D). In accordance with the Japanese Ministry of Health and Welfare guideline,4) cardiac sarcoidosis was diagnosed and a systemic glucocorticoid (prednisolone, 60 mg daily) was initiated. An ICD was also inserted as a precautionary measure for ventricular tachycardia and symptomatic complete AV block. Because the ACE level was within the normal reference range, we used 18F-FDG-PET to monitor therapy. Baseline results at >18 hours prolonged fasting showed inhomogeneous 18F-FDG uptake, accentuated in LV myocardium {maximum standardized uptake value (SUVMAX)=7.9 g/mL} (Fig. 2E and F). On discharge, the patient was asymptomatic. One month later, follow-up 18F-FDG-PET showed a marked decrease in myocardial hypermetabolism (SUVMAX=3.1 g/mL) (Fig. 2G and H), and the right paratracheal lymph node had decreased in size upon chest CT. The ACE level also dropped to 14.1 U/L. The patient is now doing well, without recurrent symptoms, including ventricular tachycardia.
Sarcoidosis is a multisystem disease that has a worldwide distribution, characterized by the presence of noncaseating granulomata in involved organs. Its etiology is currently unknown. The prevalence of this disease varies from 10 per 100000 in white populations to 35 per 100000 in African Americans.5-7) In the US, around 20-27% of patients with sarcoidosis have myocardial involvement,8) while in Japan, up to 58% of patients have cardiac lesions, accounting for as many as 85% of recorded deaths from sarcoidosis.9)
Reports also indicate that cardiac involvement is largely unrecognized antemortem. In only about 5% of patients with autopsy evidence of cardiac sarcoid (i.e., noncaseating granulomata) is cardiac disease apparent prior to death.10) Common presentations of cardiac sarcoidosis include congestive heart failure with left ventricular dysfunction, cardiac rhythm disturbance, and most importantly sudden death. The latter has been cited in up to 67% of patients where cardiac sarcoidosis was diagnosed postmortem.3)
Among the available noninvasive diagnostic modalities, gadolinium-DTPA-enhanced cardiac MRI offers greater utility for early diagnosis and follow-up, compared with the low-sensitivity of endomyocardial biopsy (approaching 20%).11-13) Recent studies have similarly demonstrated the potential of 18F-FDG-PET as a diagnostic and follow-up tool.14-16) Because the ultimate goal for these patients is to preserve cardiac function and prevent fatal arrhythmias, early detection, even in absence of symptoms, is critical. In this respect, gadolinium-DTPA-enhanced cardiac MRI and 18F-FDG-PET may more reliably facilitate early diagnosis and thus result in better treatment outcomes.14) In our patient, delayed myocardial enhancement by cardiac MRI was a key diagnostic clue. Despite procurement of an adequate endomyocardial biopsy, a tissue diagnosis could not be established with this approach. It was through EBUS-TBNA of right paratracheal and subcarinal lymph nodes that the diagnosis of cardiac sarcoidosis was eventually made.
Systemic steroids have shown survival benefits with cardiac sarcoidosis and have been generally accepted as the treatment of choice17)18) However, the effect of steroids on ventricular arrhythmias is less certain, and some recommend an ICD as primary therapy in patients presenting with ventricular tachycardia.10)19) In addition to a systemic steroid, our patient received an ICD to deal with symptomatic complete AV block and ventricular tachycardia.
Serum ACE level typically reflects sarcoid activity and can be used as a biomarker, although our patient's levels were normal. Furthermore, cardiac MRI was precluded for therapeutic monitoring, due to the precautionary ICD, so 18F-FDG-PET was used. At the one-month interval, myocardial hypermetabolism had markedly decreased by 18F-FDG-PET, which correlated well with the patient's clinical response and a corresponding decline in his serum ACE level.
In conclusion, cardiac sarcoidosis presenting with symptomatic complete AV block and sustained ventricular tachycardia, was successfully treated in this instance with a systemic steroid and an ICD, while serum levels of ACE and 18F-FDG-PET aided in therapeutic monitoring.
Figures and Tables
Fig. 1
Initial presentation of the patients. A: admission electrocardiogram: complete atrioventricular block (heart rate, 27 beats per minute). B: echocardiogram: RV and right atrium enlargement and severe RV dysfunction, without pulmonary hypertension (pulmonary artery systolic pressure, 27.64 mm Hg). C: chest X-ray: mild cardiomegaly (cardiothoracic ratio 55%); no hilar lymph node enlargement. D: Gadolinium-dietylene triamine penta-acetic acid-enhanced cardiac MRI: delayed hyper-enhancement of the entire RV wall and subepicardial (apical) or transmural (apical, basal) portions of the left ventricle. E: sustained ventricular tachycardia during endomyocardial biopsy. RV: right ventricle.
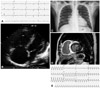
Fig. 2
Pathologic finding of lymph nodes and results of initial and follow-up 18F-FDG-PET. A and B: right paratracheal (17.27×8.35 mm) and subcarinal (18.63×11.97 mm) lymph node enlargement discovered incidentally in coronary CT angiography six months prior. C and D: lymph node aspirate: noncaseating epitheloid granulomata with giant cells. E and F: baseline 18F-FDG-PET: inhomogeneous uptake, accentuated in left ventricular myocardium {maximum standardized uptake value (SUVMAX)=7.9 g/mL}. G and H: 18F-FDG-PET at one-month follow-up; marked decrease in myocardial hypermetabolism (SUVMAX=3.1 g/mL). 18F-FDG-PET: 18F-fluoro-2-deoxyglucose positron emission tomography.
References
1. Hagemann GJ WK. Jones Williams W, Davies BH, editors. The clinical, electrocardiographic and pathological features of cardiac sarcoid. Sarcoidosis and Other Granulomatous Disorders: Proceedings of the 8th International Conference Cardiff. 1980. Cardiff: Alpha & Omega Press;601–606.
2. Johns CJ, Michele TM. The clinical management of sarcoidosis: a 50-year experience at the Johns Hopkins Hospital. Medicine (Baltimore). 1999. 78:65–111.
3. Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart: a clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med. 1977. 63:86–108.
4. Hiraga H, Yuwai K, Hiroe M. Guidelines for the Diagnosis of Cardiac Sarcoidosis: Study Report of Diffuse Pulmonary Diseases. 1993. Tokyo: Japanese Ministry of Health and Welfare;23–24.
5. Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore). 2004. 83:315–334.
6. Hillerdal G, Nöu E, Osterman K, Schmekel B. Sarcoidosis: epidemiology and prognosis: a 15-year European study. Am Rev Respir Dis. 1984. 130:29–32.
7. Rybicki BA, Major M, Popovich J Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997. 145:234–241.
8. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978. 58:1204–1211.
9. Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976. 278:455–469.
10. Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol. 1991. 18:937–943.
11. Shimada T, Shimada K, Sakane T, et al. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med. 2001. 110:520–527.
12. Vignaux O, Dhote R, Duboc D, et al. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest. 2002. 122:1895–1901.
13. Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999. 138(2 Pt 1):299–302.
14. Ohira H, Tsujino I, Yoshinaga K. 18F-Fluoro-2-deoxyglucose positron emission tomography in cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2011. 38:1773–1783.
15. Braun JJ, Kessler R, Constantinesco A, Imperiale A. 18F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging. 2008. 35:1537–1543.
16. Kim JS, Judson MA, Donnino R, et al. Cardiac sarcoidosis. Am Heart J. 2009. 157:9–21.
17. Reuhl J, Schneider M, Sievert H, Lutz FU, Zieger G. Myocardial sarcoidosis as a rare cause of sudden cardiac death. Forensic Sci Int. 1997. 89:145–153.
18. Takada K, Ina Y, Yamamoto M, Satoh T, Morishita M. Prognosis after pacemaker implantation in cardiac sarcoidosis in Japan: clinical evaluation of corticosteroid therapy. Sarcoidosis. 1994. 11:113–117.
19. Belhassen B, Pines A, Laniado S. Failure of corticosteroid therapy to prevent induction of ventricular tachycardia in sarcoidosis. Chest. 1989. 95:918–920.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download