Abstract
A young male patient diagnosed with Klinefelter syndrome was admitted to our hospital via the emergency room with chief complaints of acute chest pain and dyspnea. Pulmonary thromboembolism was diagnosed from his chest CT images. His symptoms improved after he underwent thrombolysis and anticoagulation treatment. Klinefelter syndrome has a tendency towards hypercoagulability due to hormonal imbalance and one or more inherited thromophilic factors. Thus, Klinefelter syndrome patients with a past medical history of venous thromboembolism require continuous oral anticoagulation therapy for a period of at least six months.
Klinefelter syndrome is the most common congenital abnormality causing primary hypogonadism, occurring in approximately one in a thousand (1 : 1000) live male births.1)2) This syndrome has a numerical chromosome abnormality in males, characterized by the presence of one or more extra X chromosomes.2) Affected males carry an additional X chromosome (or more), which results in abnormal development of the testis, leading to hypogonadism and infertility.3) Klinefelter syndrome has a tendency for hypercoagulability owing to the propensity for hypogonadism caused by hormonal imbalance and genetic inclination. To date, there has been no case report associated with cardiovascular disease in Korea. Here, we encountered and reported on a young male patient with Klinefelter syndrome that also had pulmonary thromboembolism.
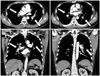
A thirty-eight year-old male patient, diagnosed as having Klinefelter syndrome (47, XXY) during the sterility test, was admitted to our hospital's emergency room with symptoms of acute chest pain and dyspnea that developed one hour prior to admission (Fig. 1). On arrival at the emergency room, his blood pressure, pulse and respiration were 90/60 mm Hg, 130/minute and 24/minute, respectively. His body temperature was 36.8℃ and he was clearly conscious. On auscultation of the chest, crackles were heard in both lower lungs. Heart sounds showed rapid pulse and no murmur. Interstitial pulmonary edema and cardiomegaly were observed from his chest X-ray, while his electrocardiography revealed findings of ST-depression in the V 3, V 4 and V 5 areas. The peripheral blood test revealed WBC 14100/uL, Hb 14.0 g/dL and platelet 465000/uL. Serobiochemical studies showed blood urea nitrogen 14.6 mg/dL, creatinine 1.2 mg/dL and hyperlipidemia was shown with total cholesterol of 210 mg/dL, triglyceride 97 mg/dL, high density lipoprotein 37 mg/dL and low density lipoprotein 177mg/dL. An immunochemical serologic test revealed a finding of inflammation with high sensitivity C-reactive protein 2.56 mg/dL. CK, creatine kinase-MB and troponin-I were all negative, while the level of D-dimer was measured to be high at 5408 ng/mL. The results of arterial blood gas analysis while the patient was on O2 mask 5 L (approximately FiO2 0.4) showed hypoxemia with pH 7.24, PCO2 28 mm Hg, PO2 62 mm Hg, bicarbonate 27 mmol/L and O2 saturation of 90%. Increased right ventricular size, decreased right ventricular function and D-shaped left ventricle were observed in the echocardiography. With a suspicion of possible pulmonary thromboembolism, contrast enhanced chest computed tomography was carried out, which revealed multiple thromboembolism in the main, lobar, segmental and subsegmental pulmonary arteries of both lungs (Fig. 2). The patient's O2 saturation dropped to 90-91% even with an O2 mask of 7 L (approximately FiO2 0.6). Owing to observations of persistent hypotension of 80/40 mm Hg despite hemodynamic support, thrombolytic therapy (Actilyse®-alteplase: 100 mg over 2 hours) was performed. Lower extremity vascular computed tomography was carried out to find the origin of venous thromboembolism. This revealed deep vein thrombosis (DVT) in the distal portion of the left popliteal vein (Fig. 3). The patient underwent anticoagulation therapy with low molecular weight heparin (Clexane®-enoxaparin: 60 mg subcutaneously every 12 hours for 7 days) and warfarin. Having observed venous thromboembolism at a young age, a screening test for immunologic diseases was carried out to make the differential diagnosis of antiphospholipid antibody syndrome. The findings of complement levels (C3, C4), antinuclear antibody (ANA), antibody to anti-double-stranded DNA, anticardiolipin antibody of immunoglobulin (Ig) M and IgG, as well as lupus anticoagulant and venereal disease research laboratory test were all negative.4) The follow-up chest computed tomography performed ten (10) days after hospital admission did not show pulmonary thromboembolism. After the patient was discharged from the hospital, he was put on warfarin for anticoagulation therapy and followed up on an outpatient basis.
There are increased incidences of venous thromboembolism in patients affected by Klinefelter syndrome. Campbell and Price5) noted increased incidences of DVT and pulmonary embolism in a series of 412 patients with Klinefelter syndrome, observed over periods ranging from 1 to 20 years. A possible explanation was provided by two consecutive studies6)7) and subsequently emphasized by Winkler8) in his review about the effects of androgens on hemostasis. According to these findings, hypogonadism in males is associated with reduced fibrinolytic activity via increased plasminogen activator inhibitor-1 (PAI-1) levels, where plasma levels show an inverse relationship with testosterone concentrations. Hemostasis is achieved by thrombin-stimulated fibrin clot formation and plasmin-induced clot lysis. PAI-1 is involved in the process of clot lysis.9) With respect to clot lysis, plasminogen, a precursor molecule for plasmin, binds to fibrin and tissue-type plasminogen activator (t-PA) to make ternary complexes. These complexes convert plasminogen into proteolytic plasmin, an active form. Here, PAI-1 suppresses t-PA, inhibiting plasmin formation, and restricts the process of fibronolysis.10)
Through such pathogenesis, patients with Klinefelter syndrome would have a higher risk of developing not just DVT but also myocardial infarction and venous thromboembolism. However, it would be difficult to explain severe thromboses with hormonal imbalance by way of hypo-androgenism alone, and one or more inherited thrombophilia {e.g., factor V Leiden and prothrombin (factor II) G20210A mutations} may be associated with it.11)
Therefore, patients with Klinefelter syndrome with a past medical or family history of venous thromboembolism should undergo an endocrinologic test, as well as additional assessment of innate or acquired thrombophilia. In the future, it seems that more studies on understanding of pathogenesis of venous thromboembolism in cases of Klinefelter syndrome are necessary. The consideration is that a long-term oral anticoagulation therapy is necessary in the treatment of thrombophilic conditions.
Figures and Tables
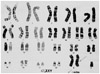
Fig. 1
Chromosomal analysis revealed a karyotype of 47, XXY, which is a typical finding for Klinefelter syndrome.
References
1. Schwartz ID, Root AW. The Klinefelter syndrome of testicular dysgenesis. Endocrinol Metab Clin North Am. 1991. 20:153–163.
2. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003. 88:622–626.
3. Ratcliffe S. Long-term outcome in children of sex chromosome abnormalities. Arch Dis Child. 1999. 80:192–195.
4. Moon HJ, Rhim CY, Kim GW, et al. Risk factors of deep vein thrombosis and pulmonary embolism in Korean. Korean Circ J. 2005. 35:474–479.
5. Campbell WA, Price WH. Venous thromboembolic disease in Klinefelter's syndrome. Clin Genet. 1981. 19:275–280.
6. Bennet A, Sie P, Caron P, et al. Plasma fibrinolytic activity in a group of hypogonadic men. Scand J Clin Lab Invest. 1987. 47:23–27.
7. Caron P, Bennet A, Camare R, Louvet JP, Boneu B, Sié P. Plasminogen activator inhibitor in plasma is related to testosterone in men. Metabolism. 1989. 38:1010–1015.
8. Winkler UH. Effects of androgens on haemostasis. Maturitas. 1996. 24:147–155.
9. Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood. 2005. 106:2605–2612.
10. Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood. 1987. 69:381–387.
11. Lapecorella M, Marino R, De Pergola G, Scaraggi FA, Speciale V, De Mitrio V. Severe venous thromboembolism in a young man with Klinefelter's syndrome and heterozygosis for both G20210A prothrombin and factor V Leiden mutations. Blood Coagul Fibrinolysis. 2003. 14:95–98.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download