Introduction
Coronary artery aneurysms (CAAs) are relatively rare but have been diagnosed recently with increasing frequency by the use of coronary angiography. The use of percutaneous coronary intervention (PCI) for lesions containing small coronary aneurysms is under controversy. Here, we report a rare case of a patient with rupture and spontaneous sealing of a coronary aneurysm that occurred within an atherosclerotic stenotic lesion after deployment of a drug-eluting stent (DES).
Case
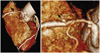
A 78-year-old man visited the cardiology outpatient department at our hospital for exertion-related chest pain that had persisted for 4 years. His only risk factor for coronary artery disease was hypertension. An electrocardiogram showed sinus rhythm and no ST-T changes. Chest radiography showed unremarkable findings. Mild diastolic dysfunction and no regional wall motion abnormalities were noted in the echocardiogram. The plasma levels of both troponin I and creatine kinase-MB were within the normal range. A 2.5-mm saccular coronary aneurysm and tubular stenosis (60-70%) was detected on the proximal right coronary artery (RCA) by using a computed tomography coronary angiography (Fig. 1).
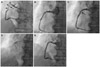
The patient was pre-loaded with clopidogrel (600 mg) and aspirin (300 mg) 1 day before the coronary angiography. His coronary angiography showed the presence of a small saccular CAA with diffuse, tubular stenosis in the proximal portion of the RCA (Fig. 2A).
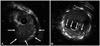
The periprocedural antithrombotic regimen consisted of a 3500-unit bolus of unfractionated heparin. A 6 Fr JR4 guiding catheter was used to engage the RCA. The lesion was pre-dilated with a 3.0×20 mm Pantera balloon (Biotronik, Berlin, Germany) at 10 atm. We then performed intravascular ultrasonography (IVUS), which showed a luminal stenosis with fibrous fatty plaque, a reference diameter of 4.5 mm, minimal lumen diameter of 1.0 mm, a post percentage diameter stenosis of 60%, and 2.5×2.5 mm sized small aneurysms in the proximal RCA (Fig. 3A). A 4.0×38 mm Endeavor® stent (zotarolimus, Medtronic, Santa Rosa, CA, USA) was implanted in the proximal RCA. After stenting, the coronary aneurysm was no longer detected, and 1 side branch of the proximal RCA was jailed. Angiography showed no luminal defect (Fig. 2B). Subsequently, adjunctive balloon dilatation using a 4.5×12 mm, non-compliant balloon was performed. Following this, acute stent thrombosis developed (Fig. 2C). We performed IVUS, which showed a thrombus in the stent, but an aneurysm was not seen (Fig. 3B). A 500 µg bolus of tirofiban was administered, followed by intravenous infusion of 0.10 µg/(kg · min). Balloon dilatation was done (Fig. 2D), follower by another 4.0×18 mm Endeavor® stent (zotarolimus, Medtronic, Santa Rosa, CA, USA) was then deployed in the thrombosis site, completely overlapping the previous stent. Immediate angiography confirmed that the intraluminal filling defect disappeared, and the Thrombolysis in Myocardial Infarction 3 flow resumed (Fig. 2E). The patient was discharged 2 days later with no additional complications. Multidetector computed tomography (MDCT) coronary angiography was performed to determine the cause of acute stent thrombosis and showed a hematoma at the initial coronary aneurysm site, suggesting that a rupture and acute sealing of the CAA due to adjunctive ballooning caused acute stent thrombosis (Fig. 4).
Discussion
Coronary artery aneurysm is defined as a coronary dilatation exceeding the diameter of normal adjacent segments or the diameter of the patient's largest coronary vessel by 1.5 times.1) The reported incidence of CAA varies from 1.5-5% with male dominance. There is a predilection for the RCA, accounting for over 40% of all cases, followed by the circumflex and left anterior descending coronary arteries.1)2)
Coronary artery aneurysms may be fusiform or saccular. Nearly 50% of patients develop CAA as a consequence of atherosclerosis.3) The natural history of CAA is mostly unknown because most of the reports in the literature include small numbers of patients with short-term follow-ups. Appropriate therapy for patients with CAA is unknown. The treatment of CAA depends on the dilatation size and existing complications. Treatment options consist of surgical, percutaneous, and medical approaches.3-5) The percutanous option has been reported as covered stent graft deployment in coronary aneurysm.6)
Few studies on the rupture of CAA have been reported, but most of these discuss spontaneous ruptures. To the best of our knowledge, there have been no reported cases on the rupture of CAA after DES stenting.7) A CAA can rupture in the pulmonary artery, right ventricle, and coronary sinus, causing an arteriovenous fistula, hematoma, or intramyocardial mass, respectively. If a CAA ruptures in the pericardial space, it can cause pericardial tamponade.7-9)
However, treatment of atherosclerotic stenotic lesions containing small CAAs has not been described previously. In our patient, we first attempted to simply implant a stent in the complex lesion, expecting that the narrow orifice of the CAA may become sealed by the expansion of the adjacent vessel wall. Unexpectedly, simple stenting with adjunctive ballooning led to immediate stent thrombosis during PCI. When an aneurysm is present, the patient is susceptible to thrombus formation for many reasons, including the stasis of blood flow, activation of various coagulation factors such as platelets, and damage to the vascular intima.1)10)11) However, in our patient, we should consider the rupture of the CAA to be a possible cause of the acute stent thrombosis because MDCT coronary angiography showed a recent hematoma near the CAA site. Low pressure simple ballooning with administration of glycoprotein IIb/IIIa inhibitors could be another treatment option, because there is no definite evidence of rupture on coronary angiogram.
There are important lessons from the present case. Firstly, PCI of stenotic lesions with CAAs should be considered for the possibility of rupture, even in cases of very small CAAs. Therefore, if it is necessary to perform PCI in lesions with small CAAs, conduct low-pressure ballooning and avoid high-pressure adjunctive ballooning, which is important because the procedure may lead to the rupture of the CAA. Secondly, we should consider that rupture and sealing of CAAs may cause immediate stent thrombosis in CAA-containing lesions. MDCT coronary angiography may be helpful to clarify the cause of immediate stent thrombosis. The best measures to repair this condition are unknown. Fortunately, another stent implantation at the acute stent thrombosis site prevented a lumen-facing thrombus growth and outward rupture expansion in the present case.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download