Abstract
Background and Objectives
Acute aortic syndrome (AAS) is a heterogeneous group of disorders that often present with severe chest or back pain. It includes acute aortic dissection (AD), intramural hematoma (IMH), dissecting aneurysm, and penetrating aortic ulcer (PAU). The clinical picture of AAS and its prognosis have not been studied in a large number of Korean patients. Therefore, we organized a multi-center registry to identify the clinical characteristics and treatment patterns, as well as long-term outcomes in Korean patients with AAS.
Subjects and Methods
Five-hundred twenty-eight patients, who had been diagnosed with AAS, were enrolled into this registry from 10 centers. On a retrospective basis, we collected demographic, laboratory, imaging data, as well as follow-up clinical outcomes by reviewing medical records from individual centers. All the data were collected in core lab and analyzed in detail.
Results
The mean patient age was 60.1±14.5 years; the male-to-female ratio was M : F=297 : 231. The prevalent risk factors for AAS included hypertension (361, 68.4%) and diabetes (52, 11.1%). The components of AAS that are included in this study are acute AD (446, 84.5%), IMH (57, 10.7%), and PAU (11, 2.1%). By type of AAS, patients diagnosed with Stanford A were 45.6% of enrolled patients, whereas those with Stanford B were 54.4% of enrolled patients. Among nearly half of the patients were treated with medicine (55.7%) alone, whereas 40.0% underwent surgery and 4.3% underwent endovascular treatment. Overall, the in-hospital event rate was 21.2% and the in-hospital death rate was 8.1%. The mean follow-up duration was 42.8 months and there showed 22.9% of total event and 10.1% of death during this period.
Acute aortic syndrome (AAS) is an acute syndrome in patients having life-threatening thoracic aortic disease. This syndrome embraces a heterogeneous group of patients with a similar clinical profile that includes classic aortic dissection (AD), intramural aortic hematoma (IMH), penetrating aortic ulcer (PAU), aortic leak, and traumatic dissection.1) Among them, AD has been regarded as a highly fatal disease despite recent advances in the diagnosis and treatment.2)3) The IMH is an increasingly recognized, potentially fatal entity with 10-30% of all AAS.4) The patient with IMH tends to be older, and most are hypertensive.5) Although less prevalent than AD or IMH, PAUs, aortic leaks, and traumatic dissections have been reported as minor components of AAS. However, there were little reports describing the clinical characteristics of AAS in the real-world populations with considerable number, especially among Koreans. So, in this study, we set out to investigate the clinical picture of AAS by collecting the data through organizing Korean multi-center registry of AAS incorporating ten centers.
From January 2000 to September 2005, 528 consecutive patients, diagnosed as having AAS from each collaborating center were retrospectively enrolled. Patients with AAS were identified by searching hospital discharge records, by surgical and echocardiographical diagnosis, and who had one of the components of AAS. Diagnosis was based on history, physical examination, image findings, and direct visualization at the time of surgery. The enrollment criteria included: 1) patients having acute chest and/or back pain; 2) pain explained by aortic origin (other causes were excluded); and 3) the image or surgical findings which coincides with the patients symptoms. The AAS included AD, IMH, PAU and the others (aortic leak and traumatic dissection). The AD was defined as a classic double channel aorta with a visible intimal tear or a flap on computed tomography (CT) scan. The IMH was defined as a crescentic or circular high-attenuation area along the aortic wall, seen without contrast-enhanced CT scan, and without evidence of flow communication through the intimal flap. The PAU was defined as a focal, contrast-filled out-pouching with irregular margins, extending beyond the expected aortic wall boundaries. For further comparison of AAS, we used Stanford classification. Unfortunately, the number of patients having PAU and the others was too small (n=25) probably due to under-reporting, we had to compare only AD and IMH, as well as Stanford type A and B AD. Differences in demographics, risk factors, presenting symptoms, treatment modality and in-hospital complications were compared in each group (AD vs. IMH; Stanford A AD vs. Stanford B AD, respectively). Patients with AD having available long-term (more than 1 year) follow-up data were also compared in Kaplan-Meier survival analysis.
The study was approved beforehand by the institutional ethics committee and the procedures followed were in accordance with the institutional guidelines. The study complied with the Declaration of Helsinki and informed consent was obtained from all patients.
A case-report form (CRF) of 100 variables, defined according to standard definitions, including demographics, history, laboratory variables, physical findings, management, imaging studies, and outcomes, was developed and used for this registry. Data were collected as CRF from each collaborating center and were forwarded to the data management center in Yonsei Cardiovascular Hospital.
All statistical analyses were performed with the use of SPSS statistics (version 18.0; Statistical Package for the Social Sciences Inc., Chicago, IL, USA). Continuous variables are expressed as mean±standard deviation. The comparison of discrete variables was done using Fisher's exact test and the comparison of means was done using the unpaired Student's t-test. Long-term adverse cardiac event were analyzed using Kaplan-Mayer survival analysis. A 2-tailed p of <0.05 was considered to be statistically significant.
As of September 2006, 528 patients were enrolled. Mean patient age was 60.05±14.51 years-old and there were more males (56.3%) enrolled. About two-thirds (n=350, 66.3%) of patients were referred from other hospitals, where the surgical approach was not capable. The components of AAS were as follows: acute AD (n=446, 84.5%), IMH (n=57, 10.7%), PAU (n=11, 2.1%) and the other (n=14, 2.6%). As risk factors of AAS, a considerable number of patients were current smokers (n=123, 57.5%) and the duration of smoking was 26.8±12.9 pack-years. The percentage of patients with diabetes was 10.6% (55 subjects). Although small in proportion, there were other risk factors, such as coronary artery disease (n=17, 3.2%), cerebrovascular disease (n=17, 3.2%), and peripheral artery disease (n=2, 0.4%) etc. Interestingly, previous history showed that 8.3% (44) of the patients also had family history of AAS. Among its etiologies, hypertension was the most prevalent (n=371, 70.3%) and the rest were like this: atherosclerosis (n=65, 12.3%), Marfan syndrome (n=21, 4.0%), trauma (n=14, 2.7%), cardiac catheterization (n=6, 1.1%), cardiac surgery (n=4, 0.8%), and pregnancy (n=2, 0.4%).
For the symptomatology, two thirds of patients presented with acute chest pain (n=394, 76.4%) within several hours, and among these patients, half of the patients had abrupt onset of pain (55.7%) on the same region. Migrating chest pain, moving from one region to another was also noted (16.7%). Other accompanied findings were pericardial effusion (on echocardiogram, 7.8%), pleural effusion (on chest X-ray, 4.4%), syncope (transient loss of consciousness, 4.0%), shock {systolic blood pressure (BP) <100 mm Hg, 3.2%}, neurologic deficit (weakness/paralysis of limb, impaired hearing/vision/speech, or loss of sensation, 2.8%), loss of consciousness (lasts more than 3 seconds, 2.8%), symptoms of heart failure (dyspnea/orthopnea/dyspnea on exertion, 1.5%) and presence of an aortic regurgitant murmur (on auscultation, 1.5%).
Approximately half of the patients had normal findings on chest X-ray (43.2%). One-third (34.8%) of patients had a widened mediastinum was only, the remaining patients had abnormal aortic contour (5.3%) and miscellaneous findings (lung or cardiac lesion, 16.7%). On electrocardiogram, two-thirds (61.0%) of the patients showed normal findings, however 17.6% showed ST-segment deviation (either horizontal elevation or depression more than 1 mm at 2 or more leads) and 21.2% showed non-specific ST-T changes. There showed no increased incidence of ST-segment deviation in patients with either coronary involvement or shock at presentation (data not shown).
The most popular diagnostic modality for AAS was CT (92.4%, 488), whereas transesophageal echocardiogram (8.1%), aortogram (4.5%) and magnetic resonance imaging (1.1%) were also performed in some institutions for the same purpose.
The AAS can be classified using well-established DeBakey and Stanford classifications. In terms of DeBakey classifications, there were class I (30.3%), class II (9.1%) and class III (60.6, most prevalent). In Stanford classifications, however, prevalence of Stanford A was a little less than Stanford B (45.6% vs. 54.4%). For the overall treatment modality, medical treatment was more than half (55.7%), and surgical (40.0%) or endovascular (4.3%) treatment were performed as well.
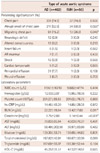
Because of the missing variables on CRF, the data from 452 patients among entire 528 patients were divided into AD (n=402) and IMH (n=50). There were no significant differences between AD and IMH group in terms of smoking status, hypertension, and diabetes. However, the age was significantly higher in IMH group than AD group (63.1±11.9 vs. 58.4±14.7, p=0.001). Although not statistically significant, there showed slight preponderance of male in IMH group than AD group (68.0% vs. 57.1%, p=0.092) (Table 1). For the characteristics of chest pain, there showed higher percentage of abrupt-onset, migratory pain in IMH group than AD group (68.0% vs. 52.5%, p=0.026; 26.0% vs. 15.2%, p=0.046). However, there were no differences in other concomitant symptoms between the 2 groups.
There was no difference in laboratory parameters between groups, except higher levels of blood urea nitrogen (BUN) and serum creatinine (Cr) in the AD group than in the IMH group, (22.22±19.64 vs. 18.42±7.60, p=0.011; 1.75±2.88 vs. 1.14±0.44, p<0.001, respectively) (Table 2).
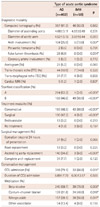
As already mentioned, over 90% of the patients were confirmatively diagnosed as AAS using CT in both group. Based on CT images, there were longer diameters of ascending aorta and aortic arch in the AD group than IMH group, without statistical significance (4.68±3.11 vs. 4.03±0.49, p=0.331; 4.52±3.16 vs. 3.59±0.44, p=0.093). Arch involvement, as well as false lumen thrombosis, were more frequent in AD group than IMH group (25.9% vs. 10.0%, 0.007; 6.9% vs. 0.0%, p=0.034). The incidence of coronary artery involvement by AAS was negligible in both groups (0.3% vs. 0.2%). The utilization of other diagnostic modalities besides CT scan as a confirmative measure, such as transesophageal/transthoracic echocardiogram, classic aortogram and cardiac magnetic resonance imaging was not different between two groups (Table 3).
In terms of AAS classifications, the percentage of Stanford A and B were similar in AD group (53.2% vs. 46.8%), whereas the percentage of Stanford B was predominant in IMH group (A: 2.0% vs. B: 98.0%). For the definitive treatment modality, the patients with AD underwent more surgical therapy than IMH patients (47.3% vs. 8.0%, p<0.001), whereas the patients with IMH was mainly treated conservatively than AD patients (86.0% vs. 48.5%, p<0.001). For the type of surgery, ascending aortic replacement was more frequently performed in AD than IMH group (34.8% vs. 6.0%, p<0.001), but in other types, there were no differences (Table 5). All the patients with IMH underwent surgery (n=4) were Stanford type A lesion. Among them, 3 patients had cardiac tamponade and 1 patient had coronary artery involvement of hematoma. All 4 patients underwent ascending aortic replacement and there were no complications regarding surgery (data not shown).
The percentage of required stay in the coronary care unit (CCU) was significantly higher for patients in the AD group rather than in the IMH group (79.1% vs. 66.0%, p=0.031). The prescription rate of beta-blockers, calcium-channel blockers, as well as intravenous nitroprussides were higher in IMH group than AD group, respectively (78.0% vs. 59.7%, p=0.008; 48.0% vs. 31.8%, p=0.018; 56.0% vs. 39.1%, p=0.017) (Table 3). The patients with IMH showed less in-hospital complications than those with AD (8.0% vs. 22.4%, p=0.010). In addition, there were no reported in-hospital death in IMH group, whereas 9.2% of patients in AD group died during hospital stay (p=0.011) (Table 4).
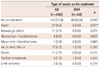
The AD group was divided further into type A AD (n=214) and type B AD (n=188) and compared. In comparison, between type A and type B AD, there showed higher prevalence of male sex, smoker and hypertension in type B than type A (65.4% vs. 49.8%, p=0.001; 45.7% vs. 35.0%, p=0.019; 72.9% vs. 61.7%, p=0.011). As an etiology of AD, type B showed higher incidence of external trauma than type A (4.8% vs. 0.9%, p=0.019). On CT-scanned images, there showed higher incidence of aortic arch involvement and pericardial effusion in type A than type B (35.9% vs. 14.4%, p<0.001; 16.4% vs. 2.1%, p<0.001, respectively) (Table 5). In terms of definitive treatment, type A underwent more surgery than type B (75.7% vs. 14.9%, p<0.001), whereas type B was managed more conservatively than type A (78.7% vs. 18.7%, p<0.001). Additionally, endovascular treatment was more utilized in type B than type A group of patients (6.4% vs. 0.9%, p=0.005).
For the type of surgery, type A group of patients underwent ascending aortic replacement, complete arch replacement and root replacement in the order of frequency, but incidence of all types of surgery is higher than type B. Accordingly, there showed higher incidence of CCU stay in type A than type B (87.9% vs. 69.1%, p<0.001). For medications, only beta-blocker was more highly used in type B than type A group (69.1% vs. 51.4%, p<0.001) (Table 5).
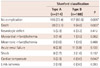
In terms of in-hospital complications, its incidence was higher in type A than type B (27.6% vs. 16.5%, p=0.005). Occurrences of in-hospital death, as well as cardiac tamponade were also significantly higher in type A than type B group of patients (13.1% vs. 4.8%, p=0.003; 2.3% vs. 0.0%, p=0.042, respectively) (Table 6).
The patients in type A group, who were not treated surgically (i.e., conservatively, n=40) seemed to have less incidence of complications, such as cardiac tamponade (2.0%), neurologic deficit (2.0%), myocardial infarct (0%), limb ischemia (2.0%) and acute renal failure (3.9%). The diameter of ascending aorta (4.96±1.19 cm), the length of total involved segment (8.73±7.30 cm), the duration of CCU stay (4.59±3.27 days) seemed to be short. The incidence of in-hospital complication was similar to overall type A group (27.3% vs. 27.6%).
On the other hand, the patients in type B group, who were treated surgically, seemed to have rather higher incidence of complications, which were neurologic deficit (5.9%), myocardial infarct (5.9%), limb ischemia (2.0%), mesenteric infarct (5.9%), and acute renal failure (11.8%). The diameter of ascending aorta seemed to be normal (3.70±0.48 cm) and the length of total involved segment was also short (7.27±5.37 cm). However, the duration of CCU stay was a bit longer than the overall type B group (9.14±7.60 vs. 6.06±4.96). On laboratory data, serum BUN, Cr, aspartate aminotransferase (AST)/alanine aminotransferase (ALT) levels were all elevated (22.1±28.8, 1.9±2.1, 113.2±214.9, 87.6±93.5, respectively), suggesting the possibility of flow compromise to the aortic branch vessels. The incidence of in-hospital complications were little higher than overall type B group (19.4% vs. 16.5%) (data not shown).
The long-term clinical follow-up was available only in 179 patients out of entire population. All the patients followed were those with Ads (type A-96 patients, type B-83 patients). Clinical and imaging follow-up duration was 42.8±36.3 months, 9.8±3.4 months, respectively. Total major adverse cardiac events (death, stroke, myocardial infarct, surgery/endovascular treatment, exacerbation of renal function) occurred in 41 patients (22.9%) and 18 patients died (10.1%) during this period. There showed trends toward shorter event-free survival for type A group than type B group (9.6±4.9 vs. 18.1±5.1 months, p=0.059). The death-free survival was significantly shorter in type A group than type B group (6.9±6.6 vs. 20.8±9.3 months, p=0.015) (Figs. 1 and 2).
The term AAS, coined by Vilacosta and associates in 1998, refers to a heterogeneous group of conditions that cause a common set of signs and symptoms, the foremost of which is aortic pain.1) Various diseases may cause this striking presentation, including trauma, pseudoaneurysm, and ruptured atherosclerotic aneurysm, but the term has come to subsume AD, intramural hematoma (IMH) and PAU.6) Despite its appearance in the literature a decade ago, there have been few reports on evaluating its nature, as well as its clinical significance. In this study, we sought to demonstrate the clinical picture of this entity in Korean patients, by the collaborative efforts of making AAS multicenter registry and we believe, to our knowledge, this report is the first in this regard.
In the literature, the international registry of acute aortic dissection (IRAD) was the first multicenter experience of collecting data in patients that had acute AD, a major component of AAS. In that registry, 12 international referral centers enrolled the data. This IRAD gave the idea of creating the Korean AAS registry.
The typical patient in IRAD registry was a male in his seventh decade with a history of hypertension who presents with abrupt onset of chest pain.7)8) In our registry, the clinical characteristics were slightly similar with IRAD: mean age 60 year-old, over 70% of the patients have hypertension and acute chest pain, as presenting symptoms. It might be explained that about three quarter of patients were having ADs in our registry, which make the clinical characteristics\similar to that of IRAD. However, unlike IRAD, the percentage of females was similar to that of males, suggesting that Korean female patients were more frequently affected by AAS than those in the Western countries.
Classically, the earlier studies emphasized the value of abnormal chest X-ray findings, among them most popular one is widened mediastinum.8)9) In our registry, about a half of the patients did not showed any evidence of mediastinal widening, which suggests that chest X-ray could not be used alone as a screening purpose for patients with AAS. As expected, the CT was the imaging modality of choice in nearly all patients (92.4%). On the contrary, aortogram and TEE, which was frequently used before, were rarely utilized as initial diagnosis.
For the treatment of AD (type A and B), overall in-hospital mortality has been reported as 27.4% in IRAD registry.2) In our study, overall in-hospital event rate was 21.1% and mortality was only 8.2% in all patients with AAS. In addition, over 80% of the patients in IRAD underwent surgery and the patients who had not underwent surgery has a other condition such as extreme age (over 80 years old) and serious comorbidity. On the contrary, our registry included patients with IMH, who are believed to have lower event rates and the number of type B dissections were similar to that of type A. In addition, unlike IRAD patients, only half of the patients in our registry underwent surgery as a definitive treatment. As we can confer from these results, the patients with different characteristics translated into a better in-hospital outcome.
It is interesting to note that the mean systolic/diastolic BP was significantly higher in IMH group than AD group, as well as type B AD group than type A AD group. Even though the BP itself in both groups was above shock range (over 100 mm Hg), the difference in BP have probably influenced the more usage of anti-hypertensive medications (especially beta-blocker and calcium channel blocker) in the former groups than the latter groups, which might have affected patient outcome to certain degree.
The decline in renal function, as demonstrated by higher level of BUN and Cr in AD group than IMH group from our registry can be explained by several causes: higher incidence of type A lesions, a higher incidence of hypotension/shock, and possibly increased renal artery involvement.
Comparison between type A and type B dissections had interesting results. For type A, two-thirds (75.7%) of the patients underwent surgery, whereas for the type B, more than two-thirds (78.7%) of the patients were treated with medicine; this can be demonstrated as approximately equal to 50% of the rate of surgery and medical management among the AD population in our study.
There were several single center reports on the surgical management of type A AD. According to the report by Cho et al.10) which showed the outcome of 28 patients after aortic arch replacement for type A AD, the hospital mortality after surgery was 3.6% and in-hospital neurologic complication was 17.9%. During the 26-month follow-up, 10.7% of patients underwent reoperation due to aortic aneurysm formation, but there was no late death. In another recent report by Campbell-Lloyd et al.11) they reported on 65 patients with acute type A AD that had undergone surgical repair. In that report, full arch replacement was performed in 14% of patients and concomitant coronary artery bypass graft was performed in 11%. Postoperative neurologic dysfunction was present in 33.8% of patients and only significant predictor of poor neurologic outcome was full arch replacement. In our registry, the percentage patients underwent complete arch replacement for type A AD was similar to the reports by Campbell-Lloyd et al.11) (14% vs. 13.6%), but interestingly enough the rate of neurologic complication was as low as 2.3%. The reason why might be due to several factors: different patient demographics, concomitant disease, the timing of surgery and the surgical skill of operators each hospital. In contrast to the traditional guidelines, there were patients with type A AD treated conservatively, as well as those with type B AD treated surgically. In our registry data shown in the result, the former group of patients had fewer complications in aortic root, arch, and comorbid conditions (acute renal failure, mesenteric infarct etc.). But, the rate of in-hospital complications was similar to the overall type A AD group, suggesting that although the patient had type A AD, if there were no or little root or arch-related complications, then we could choose medical therapy for the first strategy. The latter group of patients had a little higher incidence of complications regarding the flow compromise to the aortic branch vessels, such as mesenteric infarct, acute renal failure, as well as higher serum levels of BUN/Cr, AST/ALTs, which necessitated use of the surgical option. Because the patients in this group underwent surgery as a bail-out strategy, it seems a matter of course that the rate of in-hospital complications was higher than those with overall type B group, as shown in our registry data.
For the treatment of IMH, over 80% of the patients were treated medically and only 8% underwent surgery. The IMH has been recently recognized as a unique disease entity with pathological and clinical features different from classic AD.12-16) There are many reports that IMH is also regarded as a similar entity to classic AD in terms of disease progression and it should be treated, as similar to AD.13)16) However, there were also reports of favorable hospital outcome after medical treatment for IMH, accompanied by complete resorption of IMH itself.14)15)17) Recent report by Song et al.18) demonstrated the outcome of 357 consecutive patients presented with acute type A AAS. Among them, one third (28.3%) belonged to the diagnosis of IMH and over 80 percent of IMH patients received initial medical treatment. Interestingly enough, in-hospital mortality rate showed no difference between IMH patients and surgically treated AD patients (7.9% vs. 10.7%; p=0.56), suggesting that initial medical treatment could be a plausible option for type A IMH. In our registry data however, the majority of IMH patients were type B, which might be strong explanation for the large percentage of patients having received initial medical treatment. It is interesting to note that few selected patients with IMH who underwent surgery had aortic root involvement in our registry data (n=4). As we mentioned before, 3 patients had cardiac tamponade and 1 patient had coronary artery involvement of mural hematoma, suggesting that even though IMH has a favorable prognosis, surgical therapy should be considered if the patient had aortic root involvement.
Endovascular treatment is regarded as a well-established option for the treatment of AD. Even though total number of endovascular treatment was small, patients with type B underwent more frequently than those with type A (6.4% vs. 0.9%). Because of the recent development of endovascular equipment, among them are sophisticated stent grafts from many companies, the number of endovascular treatment certainly be increased over time. In Korea, Kang et al.19) reported the early experiences of stent graft implantation in Standford type B AD among 28 patients. In that report, clinical success rate was as high as 96.4% and there were no reported mortality regarding the procedure, as well as no mortality afterwards during 22.1 months' follow-up. A recent report by Botsios et al.20) showed the outcome of endovascular treatment for 32 patients presented with complicated acute type B dissection. In that report, the procedure was successful in all patients and in-hospital mortality seemed to be acceptable (9.3%). Although the number of patients with type B AD included in our data was relatively small (12 patients), all of the the subjects underwent successful endovascular treatment without in-hospital complications (data not shown).
In terms of vascular inflammation affecting AAS outcome, there were few reports. Lee et al.21) reported the outcome of 81 medically-treated type B AD patients in regard to the level of vascular inflammation. In that report, they demonstrated that a high monocyte count (>1250/mm3), high level of high-sensitivity C-reactive protein (hsCRP, >11 mg/dL) and D-dimer (>1.2 mg/dL) as a independent determinant of short-term mortality. In our data, the mean hsCRP for AD and IMH were 14.45 and 14.85, all of which were above cut-off applying the data by Lee et al.21) Based on that, our findings simply suggests that serum hsCRP level seemed to be high in patients with AAS, but it is uncertain whether hsCRP is an independently significant predictor of a poor outcome event or not.
There are several limitations associated with the analysis of our data. This was a multicenter registry and thereby has inherent limitations. First, because the patients were enrolled by retrospective review of medical records, selection bias might be introduced from the beginning of enrollment. Also, many patients were dropped from the analysis between each groups due to missing variables on CRF, this may have inserted further selection bias. Second, the treatment outcome of surgery is largely dependent upon the technical expertise of each operator in each hospital. So, this should be taken into consideration while analyzing the data. Third, the treatment strategy for each type AAS might be also different in each hospital (for instance, the one hospital prefer to treat medically for uncomplicated type B AD, while other prefer to perform surgery etc.). So, it is often difficult to judge one type of treatment is better than the other. Fourth, because the long-term data were only obtainable from the selected AD group, the survival analysis cannot reflect the whole AAS registry patients.
In conclusion, AAS is a group of heterogeneous diseases that have different natural history and different clinical course. By organizing real-world multicenter registry of AAS, we could demonstrate diverse features, as well as clinical impact of this rare entity in Korean patients. Further, a large-sized prospective study is warranted.
Figures and Tables
Acknowledgments
This study was supported by a grant from the Korean Society of Interventional Cardiology.
References
1. Vilacosta I, Román JA. Acute aortic syndrome. Heart. 2001. 85:365–368.
2. Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000. 283:897–903.
3. Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type A aortic dissection. Circulation. 2002. 105:200–206.
4. Vilacosta I, Aragoncillo P, Cañadas V, San Román JA, Ferreirós J, Rodríguez E. Acute aortic syndrome: a new look at an old conundrum. Heart. 2009. 95:1130–1139.
5. Coady MA, Rizzo JA, Elefteriades JA. Pathologic variants of thoracic aortic dissections: penetrating atherosclerotic ulcers and intramural hematomas. Cardiol Clin. 1999. 17:637–657.
6. Lansman SL, Saunders PC, Malekan R, Spielvogel D. Acute aortic syndrome. J Thorac Cardiovasc Surg. 2010. 140:6 Suppl. S92–S97.
7. Hirst AE Jr, Johns VJ Jr, Kime SW Jr. Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore). 1958. 37:217–279.
8. Wilson SK, Hutchins GM. Aortic dissecting aneurysms: causative factors in 204 subjects. Arch Pathol Lab Med. 1982. 106:175–180.
9. Earnest F 4th, Muhm JR, Sheedy PF 2nd. Roentgenographic findings in thoracic aortic dissection. Mayo Clin Proc. 1979. 54:43–50.
10. Cho SH, Sung K, Park KH, et al. Midterm results of aortic arch replacement in a Stanford type A aortic dissection with an intimal tear in the aortic arch. Korean Circ J. 2009. 39:270–274.
11. Campbell-Lloyd AJ, Mundy J, Pinto N, et al. Contemporary results following surgical repair of acute type A aortic dissection (AAAD): a single centre experience. Heart Lung Circ. 2010. 19:665–672.
12. Mohr-Kahaly S, Erbel R, Kearney P, Puth M, Meyer J. Aortic intramural hemorrhage visualized by transesophageal echocardiography: findings and prognostic implications. J Am Coll Cardiol. 1994. 23:658–664.
13. Nienaber CA, von Kodolitsch Y, Petersen B, et al. Intramural hemorrhage of the thoracic aorta: diagnostic and therapeutic implications. Circulation. 1995. 92:1465–1472.
14. Song JK, Kim HS, Kang DH, et al. Different clinical features of aortic intramural hematoma versus dissection involving the ascending aorta. J Am Coll Cardiol. 2001. 37:1604–1610.
15. Kaji S, Akasaka T, Horibata Y, et al. Long-term prognosis of patients with type A aortic intramural hematoma. Circulation. 2002. 106:12 Suppl 1. I248–I252.
16. Evangelista A, Mukherjee D, Mehta RH, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005. 111:1063–1070.
17. Moizumi Y, Komatsu T, Motoyoshi N, Tabayashi K. Management of patients with intramural hematoma involving the ascending aorta. J Thorac Cardiovasc Surg. 2002. 124:918–924.
18. Song JK, Yim JH, Ahn JM, et al. Outcomes of patients with acute type A aortic intramural hematoma. Circulation. 2009. 120:2046–2052.
19. Kang WC, Joung BY, Ko YG, et al. Favorable outcome of endovascular stent-graft implantation for Stanford type B aortic dissection. Korean Circ J. 2003. 33:457–464.
20. Botsios S, Schuermann K, Maatz W, Keck N, Walterbusch G. Complicated acute type B dissections: a single-center experience with endovascular treatment. Thorac Cardiovasc Surg. 2010. 58:280–284.
21. Lee S, Kim W, Hwang SH, et al. The relationship of inflammatory reaction with the mortality of type B acute aortic syndrome. Korean Circ J. 2006. 36:387–392.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download