This article has been corrected. See "Erratum: Iatrogenic Bidirectional Dissection of the Right Coronary Artery and the Ascending Aorta: The Worst Nightmare for an Interventional Cardiologist" in Volume 43 on page 434.
Abstract
Although rare, iatrogenic aortocoronary dissection is one of the complications most dreaded by the interventional cardiologist. If not managed promptly, it can have redoubted and serious consequences. Herein, we present the case of a 70 year-old woman who was treated by stenting of the second segment of the right coronary artery (RCA) for recurrent angina but, unfortunately, the procedure was complicated by anterograde dissection of the RCA with a simultaneous retrograde propagation to the proximal part of the ascending aorta. Successful stenting of the entry point was able to recuperate the RCA and to limit the retrograde propagation to the ascending aorta, but there was an extension of the dissection to the aortic valve leaflets resulting in a massive aortic insufficiency. Therefore, an isolated surgical aortic valve replacement was performed.
Iatrogenic coronary artery dissections are well known complications in the era of interventional cardiology and especially with the advances in technology, devices, and operator experience that have led to the performance of more complex percutaneous interventions. Management of the dissection propagating to the ascending aorta is challenging.1)2) We present a case of iatrogenic bidirectional dissection of the right coronary artery (RCA) and the ascending aorta that was managed rapidly by stenting the entry point of the dissection, however, the aortic valve was involved which necessitated valve replacement surgery.
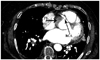
A 70 year-old woman was admitted to our hospital for evaluation of recurrent angina. She had presented with hypertension and ch-ronic atrial fibrillation. Diagnostic catheterization through the right radial artery was performed using a 6 French (6 Fr) arterial sheath and revealed a heavily calcified tight stenosis in the second segment of the RCA. We decided to perform an angioplasty on this lesion. We used a (6 Fr) Amplatz left 0.75 (AL 0.75) Launcher guiding catheter (Medtronic Inc., Minneapolis, MN, USA). After pre-dilation with the Maverick 2 2.5×20 mm balloon (Boston Scientific Company, Natick, MA, USA), we deployed the Bare Metal Stent TSUNAMI 3.5×20 mm (Terumo, Tokyo, Japan). We postdilated with a Quantum 3.5×20 mm balloon (Boston Scientific Company, Natick, MA, USA). The first angiographic control with the guide wire 0.014 with ICE hydrophilic coating (ChoICE™, Floppy LS, Boston Scientific, Natick, MA, USA) was good with no residual stenosis in the second segment of the RCA. Next, we pulled back the guide wire and the selective injection of the RCA showed a permeable RCA with a proximal dissection line (Fig. 1A) and a retrograde opacification of the proximal segment of the as cending aortic wall (Fig. 1B). In addition, we found a stagnation of contrast material within several centimeters of the aortic wall and the proximal segment of the RCA (Fig. 2A), a finding suggestive of bidirectional dissection. We immediately advanced the guide wire 0.014 and successfully stented the RCA ostia's with another Bare Metal Stent TSUNAMI 3.5×15 mm (Terumo), into the entry point of the dissection (Fig. 2B). The patient's condition following the surgical procedure remained stable. Computed tomography scans performed the same day exhibited the type A (Stanford classification) aortic dissection (Fig. 3). The cardiac ultrasound taken on the same day after the procedure showed an extension of the dissection into the aortic valve leaflets with consequent massive aortic insufficiency. An isolated aortic valve replacement was performed but the post-surgical period was complicated by the incidence of uncontrollable septic shock and death occurred at day seven following the procedure.
Iatrogenic coronary artery dissections may be caused by direct trauma from the catheter tip or through entrapment in an atheroma plaque. Other possible causes of dissection include over inflation and dilation of the balloon or stent during percutaneous coronary interventions, the dilation of a calcified plaque, the fracture of the stent strut or from aggressive handling of guide wires, specifically those that are rigid and hydrophilic. The extension of the dissection seemed to be accelerated by vigorous contrast media injections. Once coronary dissection occurs, it can rapidly propagate in the anterograde direction into the distal part of the coronary artery and, sometimes, in the opposite direction into the aorta.3) In these latter cases, if the dissection was limited to a few centimeters beyond the aortic valve, it can be treated by sealing the entry point with a coronary stent.4) Follow up after the procedure could then be performed using a CT scan or by transesophageal echocardiography5) as most of these aortic dissections heal spontaneously. Furthermore, life threatening situations may occur if the dissection is extensive and involves the ascending aorta which would necessitate urgent surgical intervention.3)6) The integrity of aortic valve should be assessed by echocardiography in all cases.
In our specifc patient, the bidirectional dissection is thought to have been related to direct trauma casued by the catheter tip with a non-coaxial alignment provoked by the removal of the guide wire 0.014 after the stent implantation. The patient was hemodynamically stable and the dissection extended only 2 to 3 cm into the ascending aorta which explains the reasoning for stenting the entry point to allow the aortic dissection to heal spontaneously.
The present case report suggests that if the patient's hemodynamic conditions were stable, and the propagation of the dissection was limited to the early proximal region of the ascending aorta, sealing the coronary entry door with a coronary stent is a reasonable strategy. Imaging surveillance should be considered for early detection of other complications and during the follow up examination. The surgical approach should be reserved for cases involving impairment of hemodynamic conditions or extensive involvement of the aorta.
Figures and Tables
Fig. 1
The dissection line (arrow) at the level of the proximal right coronary artery (A) and the retrograde opacification of the proximal segment of the ascending aortic wall (false lumen) (arrow) highlighting the aortic dissection (B).
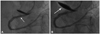
Fig. 2
The stagnation of contrast media within several centimeters of the aortic wall and the proximal segment of the right coronary artery (RCA) prior to stenting and the observed bidirectional dissection (A) and the angiographic results following the stenting procedure of the ostium of the RCA (B).

References
1. Akgul F, Batyraliev T, Besnili F, Karben Z. Emergency stenting of unprotected left main coronary artery after acute catheter-induced occlusive dissection. Tex Heart Inst J. 2006. 33:515–518.
2. Wykrzykowska JJ, Carrozza J, Laham RJ. Aortocoronary dissection with acute left main artery occlusion: successful treatment with emergent stenting. J Invasive Cardiol. 2006. 18:E217–E220.
3. Dunning DW, Kahn JK, Hawkins ET, O'Neill WW. Iatrogenic coronary artery dissections extending into and involving the aortic root. Catheter Cardiovasc Interv. 2000. 51:387–393.
4. Al-Saif SM, Liu MW, Al-Mubarak N, Agrawal S, Dean LS. Percutaneous treatment of catheter-induced dissection of the left main coronary artery and adjacent aortic wall: a case report. Catheter Cardiovasc Interv. 2000. 49:86–89.
5. Maiello L, La Marchesina U, Presbitero P, Faletra F. Iatrogenic aortic dissection during coronary intervention. Ital Heart J. 2003. 4:419–422.
6. Yip HK, Wu CJ, Yeh KH, et al. Unusual complication of retrograde dissection to the coronary sinus of valsalva during percutaneous revascularization: a single-center experience and literature review. Chest. 2001. 119:493–501.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download