Abstract
Stent migration from the delivery balloon catheter is a rare but serious complication during percutaneous coronary intervention, particularly when a part of the stent stretches into the aorta. We report an unusual case of stent migration treated with a combination of a gooseneck snare and rotablation. A part of the stent was overstretched and unrolled into the aorta and the rest of the stent remained implanted in the coronary artery. The stent was captured with a gooseneck snare but could not be retrieved because it was connected to a stent remnant implanted in the coronary artery. The stent strut was cut with rotablation, and the stent was successfully removed through the femoral sheath.
Stent migration from the delivery balloon catheter is a rare but serious complication during percutaneous coronary intervention (PCI), particularly when there are difficulties in crossing a lesion with the stent.1) When the migrated stent is trapped inside the coronary artery, stent implantation at the location of entrapment may be the safest technique.2)3) However, the stent sometimes migrates to a critical location and damages it, rendering these techniques unsuitable. In all, around 15-20% of patients with failed percutaneous retrieval of migrated stents are referred for emergency cardiac surgery that again still carries a significant perioperative risk.2)4)
In this article, we report our experience in the retrieval of the damaged intracoronary stent remnants using a combination of a gooseneck snare and rotational atherectomy.
A 61-year-old female patient presented at our clinic with complaint of exertional dyspnea and chest discomfort for about 1 year. A treadmill test showed a positive result at stage 3 (Duke score=-2).
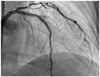
A transradial diagnostic angiography showed a diffuse 80% stenosis in the proximal-to-mid-left anterior descending artery (LAD). The lesion was calcified with mild tortuosity (Fig. 1A). A 6 Fr extra backup-3.5 guiding catheter (Medtronic, Minneapolis, MN, USA) was engaged transradially. The LAD lesion was crossed with a 0.014-inch Runthrough (Terumo, Tokyo, Japan) guidewire. The lesion was predilated with a 2.0×20 mm Nimbus balloon (Clearstream, Country Wexford, Ireland) at 14 atm. We attempted to implant an Endeavor Resolute stent (2.5×30 mm Medtronic, Minneapolis, MN, USA), but it could not pass through the proximal LAD. We used the buddy-wire technique with another Runthrough guidewire and successfully implanted the stent. The guidewires were removed to complete the procedure. However, at the end of the surgery, a coronary angiography found a type-B dissection at the distal edge of the stent (Fig. 1B).
We rewired the lesion and tried to cover the dissection with another Endeavor Resolute stent (2.5×30 mm), but found it difficult to deliver the new stent across the proximal edge of the implanted stent. We again used the buddy-wire technique with a Runthrough guidewire. The new stent passed the proximal edge of the implanted stent but was again caught in the middle of the implanted stent. We tried to push and pull the stent, but it did not move. Although we managed to successfully pull the stent-delivery balloon catheter out of the guiding catheter, an angiogram showed that a part of the stent had migrated and was positioned in the aortic sinus with a thin wire connection to the stent in the LAD (Fig. 2).
The patient was transferred to the Samsung Medical Center (SMC) immediately because our hospital had no interventional retrieval equipment or surgical backup and, fortunately, she suffered no hemodynamic deterioration during transport.
Percutaneous coronary intervention was started immediately after the patient arrived at the SMC. A left coronary angiography was obtained with a 6 Fr XB-3.5 guiding catheter (Cordis, Miami, FL, USA) through the right femoral artery. We pulled back the guiding catheter positioned in the ascending aorta to catch the stent floating in the aortic sinus. After several attempts, a part of the stent strut was captured (Fig. 3A) with a 10-mm multisnare (Microvena, St. Paul, MN, USA); however, the stent could not be pulled out even with strong traction. During traction, the operator felt resistance with each heartbeat, and the patient complained of severe chest pain. Another 7 Fr XB-3.5 guiding catheter (Cordis, Miami, FL, USA) was placed in the left coronary artery through the left femoral artery, to prepare for an emergency.
After some discussion, we decided to grind and cut out the stent strut with a rotablator (Boston Scientific, Natic, MA, USA). The rotawire was inserted into the distal LAD and across the implanted stent through the 7 Fr guiding catheter. We started the rotablation with a 1.75 mm burr (Fig. 3B) and with the captured stent pulled back with the multisnare under mild traction through a 6 Fr guiding catheter. After two rounds of rotablation in the left main artery, the stent captured (Fig. 3C) by the multisnare was released and retrieved through the sheath in the right femoral artery (Fig. 4A). Examination of the retrieved stent showed that a part of the second stent was trapped in the strut of the first stent (Fig. 4B).
However, the balloon catheter still could not be passed to the first stent, and additional rotablation had to be performed in the proximal LAD. An intravascular ultrasonography (IVUS) showed that the wire distal to the stent was in the false lumen and that the first stent was shortened to a length of -5 mm (Fig. 5A). The presence of severe dissection from the proximal stent edge to the distal left main artery was also revealed (Fig. 5B). The proximal-to-mid-LAD lesion was dilated with a 2.5×20 mm Maverick balloon (Boston Scientific, Natick, MA, USA) at 14 atm, and 3.0×28 and 3.5×28 mm Xience-V (Abbott, Santa Clara, CA, USA) stents were implanted from the proximal LAD to the left main artery. The diagonal artery was found to be occluded with Thrombolysis in Myocardial Infarction group grade 1 flow, mostly because of dissections extending from the LAD. After consideration of the risk-to-benefit ratio, the occluded diagonal artery and the dissection distal to the first stent were not treated further. The final angiogram showed an acceptable result in the LAD and the occluded diagonal arteries (Fig. 6). The patient was discharged two days after the procedure and continued as an outpatient for three months, with no reported events.
In this article, we report the case of successful removal of stent remnants entrapped in the coronary artery of a 61-year-old female patient. The remnants extended to the aortic sinus and could not be removed by a simple snare technique. We cut out the stent strut with rotational atherectomy and removed it percutaneously with a gooseneck snare.
The reported incidence of entrapment or fragmentation of devices during or after intervention ranges from 0.2-0.8%.5) The initial treatment for intravascular foreign objects used to be surgery. However, with the development of PCI, the primary treatment is now endovascular retrieval.3) As of now, no guidelines exist for optimal surgical management other than successful nonsurgical snaring and retrieval.3) This complication with catheter or stent remnants that are entrapped during PCI is life threatening because it can lead to myocardial ischemia, infection, thrombosis, and perforation.
Stent migration has been reported in 0.3-1.2% of PCI procedures.1)2) In cases of misplaced stents, these can either be retrieved or can be implanted in another vascular site. The technique chosen depends on the characteristics of the stent, whether the stent is of a balloon or self-expanding type, and on the site of stent displacement. If it is possible to reimplant the stent at a suitable site, then reimplantation should be the primary rescue technique because retrieval can lead to vascular trauma.3)
In the case under study in this article, a part of the stent was found in the aortic sinus, and hence, reimplantation of the stent was not possible. First, we selected a snare loop for retrieval of stent remnants. Percutaneous retrieval of foreign bodies is a well-established procedure, with an excellent success rate and a low complication rate. Sheth et al.,3) in their study, reported that 96.2% of foreign objects were successfully removed with Dormia baskets, while Wolf et al.6) showed that a snare loop was successfully used for foreign object removal in 87% of cases. They suggested that the endovascular management of lost or misplaced intravascular objects, particularly using the Amplatz GooseNeck® snare, is highly effective with only a few minor complications. The occurrence of stent remnants floating in the aortic root, as observed in this case, is rare. In our case, embolization of the wire itself was unlikely, but a thread of stent strut extending from the LAD into the aortic root across the left main artery could have caused a fatal thrombosis.
The mechanism by which the second stent was trapped by the first stent is not clear. We assume that the guidewire undermined the proximal edge of the first stent during rewiring in order to reenter the distal LAD. The second stent traveled over the misplaced guidewire and was undermined in the cell at the proximal edge of the first stent. After rotablation, we removed not only the second stent but also a part of the first stent. Angiogram and IVUS showed that the first stent was shortened after removal. The Endeavor® stent has two connections between segments that could possibly contribute to the overstretching and extension of the struts, as observed in this case. The most probable cause of the proximal dissection in this case would be a damage that had taken place while the entrapped stent was being withdrawn. Another possible explanation would be an injury caused by the dislodged stent while it was passing through the proximal LAD. On the basis of the operator's opinion, it was concluded that it was less likely that the dissection had resulted from either the procedure of rotablation or rewiring.
Traction alone did not work for withdrawal of the stent remnants because the migrated stent was connected to the stent that was already implanted in the LAD, so we eventually used rotational atherectomy to grind and cut out the stent strut. Following the initial rotablation on the dislodged stent, one more rotablation was performed to ablate the remaining fragment of the cut stent. Using this two-step rotablation, the balloon catheter was pushed forward successfully. During the second stenting, we believed that the wire was placed outside the previously implanted stent, which allowed the second stent to undermine the first one through between stent struts. One should be more cautious not to allow the second stent to undermine the first one.
This article is the first reported case of retrieval of intravascular stent remnants using a combination of rotational atherectomy and a gooseneck snare. There were no earlier reports on the utilization of rotational atherectomy for the retrieval of entrapped stents.
Figures and Tables
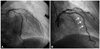 | Fig. 1The comparison of the mid-left anterior descending artery pre-stenting and post-stenting. A: left coronary angiogram (anterior-posterior cranial view) showed a calcified tortuous bifurcation lesion at the mid-left anterior descending artery. B: left coronary angiogram (right anterior oblique cranial view) demonstrating a long type-B edge dissection at the distal part of the stent (arrows). |
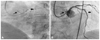 | Fig. 2The migrated stent in the aortic sinus. A part of it is in the aorta (thin arrow) and the rest inside the left anterior descending artery (thick arrow) with a connection to the thread of the stent strut that is unrolled and outstretched. A: angiographic anterior-posterior cranial view. B: fluoroscopic left anterior oblique cranial view. |
 | Fig. 3Fluoroscope photos to illustrate the porcedure in removal of the remnant stent. A: a part of the stent in the aortic sinus was captured by a 10-mm multisnare. B: grinding and curing out the stent strut by rotablator (thin arrow) with a 1.75 mm burr, and the remnant stent inside the left anterior descending artery (thick arrow). C: the stent captuted by the multisnare was released through the sheath in the right femoral artery. |
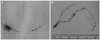 | Fig. 4The removed stent. A: the stent strut was overstretched and extended. B: two stents tangled together. |
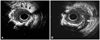 | Fig. 5Intravascular ultrasonography image taken after the removal of the stent. A: the proximal edge of the stent: part of the stent strut is missing (white arrows for stent strut, black arrows for arterial wall without stent struts). B: the presence of severe dissection (white arrow) from the proximal stent edge to the distal left main artery. |
References
1. Eggebrecht H, Haude M, von Birgelen C, et al. Nonsurgical retrieval of embolized coronary stents. Catheter Cardiovasc Interv. 2000. 51:432–440.
2. Alexiou K, Kappert U, Knaut M, Matschke K, Tugtekin SM. Entrapped coronary catheter remnants and stents: must they be surgically removed? Tex Heart Inst J. 2006. 33:139–142.
3. Sheth R, Someshwar V, Warawdekar G. Percutaneous retrieval of misplaced intravascular foreign objects with the Dormia basket: an effective solution. Cardiovasc Intervent Radiol. 2007. 30:48–53.
4. Reidemeister JC, Wolfhard U. Direct coronary bypass operation in complicated coronary dissection. Z Kardiol. 1996. 85:Suppl 1. 67–72.
5. Capuano F, Simon C, Roscitano A, Sinatra R. Percutaneous transluminal coronary angioplasty hardware entrapment: guidewire entrapment. J Cardiovasc Med (Hagerstown). 2008. 9:1140–1141.
6. Wolf F, Schernthaner RE, Dirisamer A, et al. Endovascular management of lost or misplaced intravascular objects: experiences of 12 years. Cardiovasc Intervent Radiol. 2008. 31:563–568.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download