Abstract
There have been a growing numbers of patients diagnosed with malignancy and coronary artery disease simultaneously or serially. In the era of percutaneous coronary intervention (PCI), stent thrombosis has been a rare but challenging problem. Recently, we experienced two unique cases of acute stent thrombosis in patients with malignancy. The first case showed acute and subacute stent thrombosis after PCI. The second case revealed simultaneous thromboses in stent and non-treated native coronary artery. We believe that we need rigorous precautions in the treatment of patients with coronary artery disease and malignancy, especially with regards to deciding how and whether to revascularize, as well as which anti-platelet agents to select.
Stent thrombosis is one of the most fatal complications of percutaneous coronary intervention (PCI). Even with optimal medical treatment after drug eluting stent or bare metal stent (BMS) insertion, about 0.5-1% of patients experience acute, subacute, late, or very late stent thrombosis with a mortality rate as high as 45%.1)2) Lesion-related risk factors for stent thrombosis include bifurcation lesions, longer lesions, and under deployment of the stent; patient-related risk factors include diabetes, renal failure, resistance to aspirin or clopidogrel, left ventricular dysfunction (low ejection fraction), younger age, and premature antiplatelet therapy discontinuation.1)2) Malignancy is considered a prothrombogenic condition, with increased risk for both venous and arterial thrombosis, including native coronary artery thrombosis and myocardial infarction.3) Recently, we experienced two confirmed cases of acute stent thrombosis in patients with underlying malignancy. Here we describe two cases of acute stent thrombosis with unique features.
A 56-year-old male was admitted to our center with an abnormal finding on low dose screening chest CT scan which demonstrated an atelectasis and abrupt narrowing in the right upper lobar bronchus (Fig. 1A). Bronchoscopic biopsy revealed a squamous cell carcinoma obstructing the right upper bronchus and [18F]fluorodeoxyglucose positron emission tomography demonstrated hypermetabolism in the bronchus and right lower paratracheal lymph node. Clinical staging was cT1aN2M0. The patient was scheduled for curative resection of the lung cancer. He had a past medical history of hypertension and unstable angina, which had been treated by plain balloon angioplasty 8 years previously. He was taking aspirin 100 mg daily, diltiazem 90 mg daily, isosorbide mononitrate 20 mg twice daily, and candesartan 16 mg daily, but still complained of intermittent chest pain on exertion (CCS class II). A myocardial perfusion scan showed reversible perfusion defects in the apex and the apical anterior wall suggesting left anterior descending artery (LAD) territory ischemia. Echocardiography showed basal inferior wall akinesia and basal inferolateral wall hypokinesia with a normal left ventricular ejection fraction (EF). The patient was treated with aspirin 300 mg and clopidogrel 600 mg in preparation for coronary angiography (CAG). CAG revealed 3-vessel disease with an nearly occluded proximal LAD, 75% stenosis in the proximal left circumflex artery (LCX) and diffuse 50% stenosis in the right coronary artery (RCA) (Fig. 1B). We decided to revascularize the critical ischemia before surgery. We performed PCI for the proximal LAD and the proximal LCX using BMSs (Coroflex Blue®, B. Braun Corporation, Melsungen, Germany; 3.0×19 mm and 2.75×13 mm in LAD and Driver®, Medtronic Cardiovascular, Minneapolis, MN, USA; 3.0×30 mm in LCX) (Fig. 1C). Twenty minutes after completion of the procedure, the patient developed an urticarial rash on his trunk, diaphoresis, chest pain, hypotension, and bradycardia. Radio-contrast induced anaphylactic shock was suspected. Despite medical therapy, the symptoms persisted and an electrocardiogram (ECG) showed ST-segment elevations in V 4, V 5, and V 6 (Fig. 1D). Emergent angiography with support of an intra-aortic balloon pumping demonstrated thrombotic total occlusion of the LCX stent and some thrombi in LAD stent (Fig. 1E). The stent was reopened with aspiration thrombectomy and additional ballooning, accompanied by intravenous abciximab infusion. The patient made a good recovery and was asymptomatic when discharged with aspirin 100 mg and clopidogrel 75 mg daily. He returned within the month for neo-adjuvant chemotherapy and video assisted thoracic surgery. Fifteen days later, he was re-admitted for neo-adjuvant chemotherapy (paclitaxel+carboplatin). After 10 minutes of paclitaxel infusion, he developed diaphoresis, dyspnea and chest pain with hypotension. An ECG showed ST-segment elevation of about 4 mm in V 2-V 4. Emergent angiography demonstrated intraluminal thrombi in the LAD and LCX stents (Fig. 1F). Abciximab was administered and aspiration thrombectomy and balloon angioplasty were performed, resulting in restoration of Thrombolysis in Myocardial Infarction grade 3 flow. The patient again showed a good recovery and was asymptomatic when discharged with triple anti-platelet therapy due to the recurrent episode of stent thrombosis (aspirin 100 mg and clopidogrel 75 mg daily, cilostazol 100 mg twice daily). At 42 days post-PCI, the patient underwent right upper lobectomy without any further adverse events. He is now doing well with aspirin 100 mg daily.
A 68-year-old male presented with a three-day history of fever and abdominal pain. His past medical history included hypertension and 3-vessel coronary artery disease, which had been treated by PCI 13 years previously. The medical record of the PCI was unavailable. The patient had been taking aspirin 100 mg daily along with an angiotensin converting enzyme inhibitor and a beta-blocker since the PCI. A CT scan of the abdomen revealed a pericolic abscess with pneumoretroperitoneum at the right perirenal space and a pancreatic tail mass invading into the left kidney (Fig. 2A), the spleen, and the descending colon. An acute perforated appendicitis or diverticulitis was suspected and the patient underwent emergent laparotomy. Surgical findings included an intra-abdominal abscess with severe adhesions and multiple seeding nodules on the rectal shelf and the omentum. Pathology confirmed metastatic adenocarcinoma originating from the pancreas. At postoperative day 22, despite resolution of the intra-abdominal infection, the patient developed pulmonary edema with respiratory failure and shock requiring mechanical ventilation. His ECG showed no change but cardiac enzymes were elevated (peak troponin I was 0.219 ng/mL). Echocardiography showed regional wall motion abnormality in the RCA territory and left ventricular dysfunction (EF 42%). Cardiogenic shock due to myocardial ischemia was suspected. CAG revealed a near total occlusion in the proximal LAD (Fig. 2B) and a 90% stenosis of the posterolateral branch. A 3.5×23 mm Genous™ stent (OrbusNeich, Hong Kong) was deployed in the proximal LAD (Fig. 2C) and a 2.75×18 mm Xience stent (Abbot Vascular, Abbot Laboratories, Abbot park, IL, USA) was inserted in the posterolateral branch.
Twenty minutes after PCI, the patient developed ventricular fibrillation with pulseless ventricular tachycardia (Fig. 2D), which was initially unresponsive to electrical cardioversion and anti-arrhythmic drugs. We instituted extracorporeal membrane oxygenation (ECMO) and on repeat angiography we discovered a total occlusion of the proximal LAD stent with an extensive thrombus (Fig. 2E). Abciximab was administered intravenously, and aspiration thrombectomy with additional ballooning was performed. The patient had also developed a new thrombus in the distal RCA that had not been seen during the previous angiography (Fig. 2F). A 3.0×23 mm Xience stent was placed in the distal RCA lesion because there was significant residual stenosis after plain balloon angioplasty. Once the total occlusion of the proximal LAD was reopened, the patient's ventricular tachycardia was converted to sinus rhythm. Four days later, he was weaned from ECMO support and started showing gradual recovery from pulmonary edema and cardiogenic shock. Nonetheless, the patient died from the progression of his cancer in forty days.
We have described 2 patients with acute coronary stent thrombosis, presenting with cardiogenic shock or cardiac arrest accompanied with lethal arrhythmia in the presence of concomitant malignancy.
In the first case, acute and subacute stent thrombosis occurred sequentially in the same patient. Episodes of acute hemodynamic instability, the first due to suspicious anaphylactic shock caused by radio-contrast dye and the second occurring during infusion of paclitaxel, were antecedent to the acute and subacute stent thromboses. Although acute hemodynamic instability is frequently encountered in acute coronary syndrome (ACS), acute stent thrombosis is very rare. In the second case, the patient had mild left ventricular dysfunction (EF 42%) as a risk factor for stent thrombosis.1)2) However, in this case, the thrombosis occurred not only in the stent but also in an untreated native coronary artery segment. In our experience, both of these cases were thought to be very unusual. Since malignancy is known to be a prothrombotic condition,3) we think that the patients' concomitant malignancy were one of the precipitating factors to their acute stent thromboses.
Malignancy is a well-known risk factor for deep vein thrombosis, pulmonary embolism and even arterial thromboembolism.3)4) There are several reports on cases of ACS, myocardial infarction and stent thrombosis in patients with malignancy. Tumor-produced procoagulants, inflammatory cytokines, decreased inhibitors of coagulation, impaired fibrinolysis, and general responses of the host to the tumor (i.e. acute phase reactant, angiogenesis, inflammation) may all contribute to this prothrombotic tendency.5) In addition, anti-cancer therapy such as surgery, chemotherapy, radiotherapy, and hormone therapy,6) along with hemodynamic compromise (i.e., stasis) may amplify the prothrombotic tendency in patients with cancer. ACS and myocardial infarction have also been reported during infusion of various chemotherapeutic agents, including 5-FU,7) capecitabine,8) gemcitabine,9) and paclitaxel.10) Even though the main mechanism of acute coronary syndrome with these chemotherapeutic agents is unclear, some authors have proposed coronary vasospasm or endothelial dysfunction as a cause.7)9) However, there has been no report on recurrent stent thrombosis in a patient or on simultaneous thromboses in stent and native coronary artery to the best of our knowledge. Therefore, these cases raise additional caution on PCI in patients with malignancy.
Although there is currently no statistically proven causal relationship between malignancy and stent thrombosis or ACS, increasing numbers of case reports imply that malignancy may be one of the precipitating factors of stent thrombosis. Further study with statistical power will be needed to clarify this problem. Meanwhile, we believe that we need rigorous precautions in the treatment of pa-tients with coronary artery disease and malignancy, especially with regards to deciding how and whether to revascularize, as well as which anti-platelet agents to select (i.e. triple anti-platelet therapy11) instead of newly developed agents such as ticagrelor12) and prasugrel13)).
Figures and Tables
Fig. 1
A case of recurrent stent thrombosis. A: a chest CT shows atelectasis and abrupt narrowing in right upper lobar bronchus due to squamous cell carcinoma (arrow). B: AP view; LAD was nearly occluded (arrow head). Proximal LCX shows a significant stenosis (arrow). C: AP cranial view; LAD (arrow head) and LCX (arrow) were successfully revascularized. D: electrocardiogram at the event showed ST-segment elevations in V 4, V 5, and V 6. F: AP view; emergent angiography demonstrated intraluminal thrombi in the LAD (arrow head) and LCX stents (arrow). E: AP view; emergent angiography demonstrated thrombotic total occlusion of the LCX stent (arrow) and some thrombi in LAD stent (arrow head). AP: anterioposterior, LAD: left anterior descending artery, LCX: left circumflex artery.
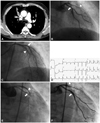
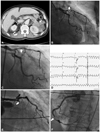
Fig. 2
A case of simultaneous acute thrombosis in stent and native coronary artery. A: a CT scan of the abdomen reveals a pericolic abscess with pneumoretroperitoneum at the right perirenal space and a pancreatic tail mass invading into the left kidney (arrow). B: anterioposterior view; coronary angiography reveals a near total occlusion in the proximal LAD (arrow) which triggered intractable cardiogenic shock in this patient. C: right anterior oblique caudal view; the lesion was successfully revascularized (arrow). D: electrocardiogram at the event shows pulseless ventricular tachycardia. LAD: left anterior descending artery. E: emergency coronary angiography at the event identified a total occlusion of the proximal LAD stent with an extensive thrombus (arrow). F: left anterior oblique view; the patient had also developed a new thrombus in the distal right coronary artery that had not been seen during the previous angiography (arrow).
References
1. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting st-ents. JAMA. 2005. 293:2126–2130.
2. Blich M, Zeidan-Shwiri T, Petcherski S, Osherov A, Hammerman H. Incidence, predictors and outcome of drug-eluting stent thrombosis in real-world practice. J Invasive Cardiol. 2010. 22:461–464.
3. Lee AY. Thrombosis in cancer: an update on prevention, treatment, and survival benefits of anticoagulants. Hematology Am Soc Hematol Educ Program. 2010. 2010:144–149.
4. Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009. 27:4839–4847.
5. De Cicco M. The prothrombotic state in cancer: pathogenic mechanisms. Crit Rev Oncol Hematol. 2004. 50:187–196.
6. Nakagawa T, Yasuno M, Tanahashi H, et al. A case of acute myocardial infarction: intracoronary thrombosis in two major coronary arteries due to hormone therapy. Angiology. 1994. 45:333–338.
7. Canale ML, Camerini A, Stroppa S, et al. A case of acute myocardial infarction during 5-fluorouracil infusion. J Cardiovasc Med (Hagerstown). 2006. 7:835–837.
8. Wijesinghe N, Thompson PI, McAlister H. Acute coronary syndrome induced by capecitabine therapy. Heart Lung Circ. 2006. 15:337–339.
9. Kalapura T, Krishnamurthy M, Reddy CV. Acute myocardial infarction following gemcitabine therapy: a case report. Angiology. 1999. 50:1021–1025.
10. Gemici G, Cinçin A, Değertekin M, Oktay A. Paclitaxel-induced ST-segment elevations. Clin Cardiol. 2009. 32:E94–E96.
11. Lee SP, Suh JW, Park KW, et al. Study design and rationale of 'Influence of Cilostazol-based triple anti-platelet therapy on ischemic complication after drug-eluting stent implantation (CILON-T)' study: a multicenter randomized trial evaluating the efficacy of Cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease. Trials. 2010. 11:87.
12. Gurbel PA, Bliden KP, Butler K, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the respond study. Circulation. 2010. 121:1188–1199.
13. Montalescot G, Wiviott SD, Braunwald E, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009. 373:723–731.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download