Abstract
Background and Objectives
Although generic clopidogrel is widely used, clinical efficacy and safety between generic and original clopidogrel had not been well evaluated. The aim of this study was to evaluate the clinical outcomes of 2 oral formulations of clopidogrel 75 mg tablets in patients with coronary artery disease (CAD) undergoing drug-eluting stent (DES) implantation.
Subjects and Methods
Between July 2006 and February 2009, 428 patients that underwent implantation with DES for CAD and completed >1 year of clinical follow-up were enrolled in this study. Patients were divided into the following 2 groups based on treatment formulation, Platless® (test formulation, n=211) or Plavix® (reference formulation, n=217). The incidence of 1-year major adverse cardiovascular and cerebrovascular event (MACCE) and stent thrombosis (ST) were retrospectively reviewed.
Results
The baseline demographic and procedural characteristics were not significantly different between two treatment groups. The incidence of 1-year MACCEs was 8.5% {19/211, 2 deaths, 4 myocardial infarctions (MIs), 2 strokes, and 11 target vessel revascularizations (TVRs)} in Platless® group vs. 7.4% (16/217, 4 deaths, 1 MI, 2 strokes, and 9 TVRs) in Plavix® group (p=0.66). The incidence of 1-year ST was 0.5% (1 definite and subacute ST) in Platless® group vs. 0% in Plavix® group (p=0.49).
Dual anti-platelet therapy with clopidogrel plus aspirin has become the standard treatment for the reduction of ischemic events in patients with coronary artery disease (CAD) with stenting, especially those undergoing drug-eluting stent (DES) implantation.1-4) However, the high cost of clopidogrel has been cited as a factor in the premature discontinuation of anti-platelet therapy causing thrombotic events in the DES era. Therefore, different formulations and generics of clopidogrel are used in an effort to reduce costs.5) Also, in Asian and South American countries, several generics versions of clopidogrel have been brought to the market.6) Recent studies have reported that original clopidogrel and generic clopidogrel were tolerable and similar in their pharmacokinetic and pharmacodyna-mic properties,7)8) but there is not enough data evaluating the clinical efficacy and safety between original clopidogrel and generic clopidogrel.
Therefore, the aim of this study was to evaluate the clinical outcomes of 2 oral preparations of clopidogrel 75 mg tablets in patients with DES implantation to determine the clinical efficacy and safety of generic clopidogrel, Platless®.
This was a retrospectively designed study. A total of 612 patients diagnosed with CAD and undergoing percutaneous coronary intervention (PCI) with DES implantation at Gachon University, Gil Hospital, between July 2006 and February 2009 were screened in this study. Inclusion criteria were patients with CAD and DES implantation who were treated with a single preparation of clopidogrel during the study period irrespective of age and diagnosis. Patients with both acute coronary syndrome and chronic stable angina were included. Exclusion criteria were patients treated with more than two preparations of clopidgrel during the study period, as well as patients that did not complete >1 year clinical follow-up.
Patients were divided into the following 2 groups based on treatment preparation, Platless® (test formulation, Sam Jin Pharmaceutical Co. LTD, Seoul, Korea) or Plavix® (reference formulation, Sanofi-Aventis, Paris, France). Before June 2008, all the patients were prescribed Plavix® for clopidogrel and since then, all the patients were prescribed Platless® for clopidogrel, because Plavix® was totally replaced by the new generic clopidogrel, Platless® at our institute.
One hundred and sixty-two patients that were treated with both Plavix® and Platless® during the study period were excluded from the study. Nine patients in the Plavix® group and 13 patients in the Platless® group did not complete >1 year of clinical follow-up, so they were also excluded. Finally, four hundred and twenty-eight patients were enrolled; 211 patients in the Platless® group and 217 patients in the Plavix® group, respectively (Fig. 1). The protocol was approved by the Institutional Review Board at Gil Hospital and written informed consent was obtained from all patients.
Percutaneous coronary intervention was performed according to standard techniques.9) Pre- and post-dilatation, selection of stent type and size were at the discretion of the physician performing the procedure. During the procedure, intravenous heparin was given to maintain an activated clotting time between 200 and 300 seconds. The use of peri-procedural glycoprotein IIb/IIIa inhibitors was left entirely to the discretion of the treating physician.10) Angiographic procedural success was defined as successful implantation of the stent into the target lesion with residual stenosis <20% and a Thrombolysis in Myocardial Infarction 3 flow at the conclusion of the procedure in the absence of dissection or thrombosis.
Prior to PCI, all the patients received aspirin (300 mg as a loading dose, followed by 100-200 mg/day) and clopidogrel (600 mg as a loading dose, followed by 75 mg/day). Aspirin was continued indefinitely and clopidogrel for at least 12 months after DES implantation. Patients with non-ST elevation acute coronary syndrome that ultimately undergo PCI and are determined to be at a high risk for ischemic complications, for instance, those with elevated troponin I or T, ST-segment depression or diabetes mellitus, as well as evidence of high intracoronary thrombus burden were administered cilostazol (100 mg bid) in addition to dual oral anti-platelet therapy by the attending physician's discretion.
Follow-up coronary angiography was performed only for patients developing recurrent symptoms or positive stress testing during the follow-up period. In the case of follow-up, coronary angiography was performed, and the decision to perform further revascularization for the target vessel was based on clinical justification.
The primary endpoint was a major adverse cardiovascular and cerebrovascular event (MACCE) after 1 year. MACCE was defined as a composite of the incidence of death, myocardial infarction (MI), stroke, and target vessel revascularization (TVR). The secondary endpoints were individual components of the primary endpoint and the incidence of stent thrombosis (ST).
Death was defined as mortality from any cause (cardiovascular or non-cardiac). MI was defined as troponin elevation with electrocardiographic changes or angina, but procedure-related MI was excluded as an endpoint. Stroke was defined as a new focal neurologic deficit of vascular origin lasting at least 24 hours. Stroke was further classified as an intracranial hemorrhage or cerebral infarction (if a computed tomographic or magnetic resonance imaging scan was available). TVR was defined as any repeated PCI or surgical bypass of any segment within the entire major coronary vessel that was proximal and distal to a target lesion, including upstream and downstream branches, and the target lesion itself. ST was defined by the Academic Research Consortium definition.11)
Clinical events were retrospectively reviewed by 2 independent physicians who were blinded to the treatment groups.
All of the continuous variables are presented as the mean±standard deviation, and compared by independent t-tests for the normally distributed variables. The categorical variables are expressed as the number of subjects or percentages and analyzed by a chi-square test or Fisher's exact test as appropriate. Statistical analysis was performed with Statistical Package for the Social Sciences (SP-SS) for Windows 12.0 (SPSS Inc., Chicago, IL, USA). P<0.05 were considered statistically significant.
The baseline characteristics of the patients included in this study are listed in Table 1. The two groups were similar with respect to age, gender, and the incidence of hypertension, diabetes, or smoking history. Lipid profiles were similar in both groups. Also, the diagnoses on admission were similar in both treatment groups; 70 patients (32.3%) in the Plavix® group and 86 patients (40.7%) in the Platless® group were diagnosed with MIs. The Plavix® group, however, had a higher incidence of prior PCIs (p=0.02).
With the exception of calcium channel blockers (CCBs), discharge medications (aspirin, beta-blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, cilostazol, and statins) were not significantly different between the two treatment groups (Table 2). CCBs were prescribed more often in the Plavix® group than the Platless® group (58.1% vs. 48.3%, p=0.04). Twenty-one patients (9.7%) in the Plavix® group and 33 patients (15.6%) in the Platless® group received triple anti-platelet therapy (aspirin, clopidogrel, and cilostazol; p=0.06); the duration of triple anti-platelet therapy was not significantly different between the Plavix® and Platless® groups (18.4±16.3 months vs. 13.3±9.1 months, p=0.24).
The mean stent diameter was 2.99±0.45 and 3.03±0.45 mm in the Plavix® and Platless® groups, respectively (p=0.30). The total stent length in the Plavix® group was longer than the Platless® group (24.29±5.45 vs. 22.81±5.97 mm, p=0.01). The average number of stents used during the index PCI was 1.5±0.9 for the Plavix® group and 1.4±0.8 for the Platless® group (p=0.11) (Table 3).
In-hospital death occurred in 1 patient (0.5%) in the Platless® group, who died due to aggravation of heart failure and a rhythm disturbance. MI occurred in 1 patient (0.5%) in the Plavix® group. Stroke occurred in 1 patient (0.5%) in the Platless® group due to a cerebral infarction. There were no in-hospital TVRs or STs in either treatment group. Taken together, the incidence of in-hospital MACCE was similar between the 2 treatment groups {0.5% (1 MI) in the Plavix® group vs. 0.9% (1 death and 1 stroke) in the Platless® group; p=0.62} (Fig. 2).
One-year deaths occurred in 4 patients (1.8%) in the Plavix® group and 2 patients (0.9%) in the Platless® group (p=0.69). In the Plavix® group, all of the deaths were due to non-cardiac causes. In the Platless® group, one patient died due to aggravation of heart failure in a state of hospitalization and one patient died due to sudden cardiac collapse. MI occurred in 1 patient (0.5%) in the Plavix® group and 4 patients (2.0%) in the Platless® group (p=0.21). Strokes occurred in 2 patients (0.9%) in the Plavix® group and 2 patients (0.9%) in the Platless® group (p=1.00). The two strokes in the Plavix® group were due to a cerebral infarction and cerebral hemorrhage, whereas both strokes in the Platless® group were due to cerebral infarction. TVRs occurred in 9 patients (4.1%) in the Plavix® group and 11 patients (5.2%) in the Platless® group (p=0.60). Taken together, the incidence of 1-year MACCE was similar between the 2 treatment groups {7.4% (4 deaths, 1 MI, 2 strokes, and 9 TVRs) in the Plavix® group vs. 8.5% (2 deaths, 4 MIs, 2 strokes, and 11 TVRs) in the Platless® group; p=0.66} (Fig. 3). Except for cerebral hemorrhage, one case of gingival bleeding occurred in Plavix® group and one duodenal ulcer bleeding that improved without intervention occurred in Platless® group.
Stent thrombosis occurred in 1 patient (0.5%) in the Platless® group (p=0.49). The ST in the Platless® group was a definite and subacute event (Table 4). Initially, the patient was admitted and diagnosed with a non-ST elevation MI and underwent PCI with 2 long DESs (total stented length, 66 mm) at the proximal-to-mid left anterior descending artery. The patient was medicated with a triple anti-platelet regimen (aspirin, clopidogrel, and cilostazol). Seven days after procedure, the patient complained of severe chest pain suddenly. Emergent coronary angiography revealed total thrombotic obstruction of a previous stent at the left anterior descending artery, which was treated successfully.
Our data showed that the incidence of in-hospital and 1-year MACCE was similar in the Plavix® and Platless® groups. The incidence of ST was very low, and similar between the two treatment groups. This is the first study, to our knowledge, that compares the clinical events demonstrating efficacy and safety between generic clopidogrel and original clopidogrel in patients that had CAD and underwent PCI with DES.
There is limited data available on the clinical events of generic clopidogrel and original clopidogrel. Only a few reports have compared the pharmacokinetic or pharmacodynamic profiles of generic clopidogrel and original clopidogrel.7)8)12-14) A prospective, controlled study in Koreans among patients on Plavix® maintenance therapy with coronary stents, replacement with Plavitor®, a generic clopidogrel bisulfate, showed comparable inhibition of adenosine diphosphase-induced platelet aggregation.13) A randomized, open-label, crossover study in healthy Korean male subjects revealed bioequivalence and tolerability of two different clopidogrel salt formulation, besylate (Plavid®) and bisulfate (Plavix®).7) However, a study on the analysis of purity in 19 drug product tablets containing clopidogrel, in which there were 18 copies of clopidogrel bisulfate versus the original brand (Plavix®), showed that most of the formulations were not of equivalent quality compared to the original drug product; specifically, the amount of impurities was higher, the content of clopidogrel was lower, and the dissolution profiles were different in the generic formulations.6)
A meta-analysis showed that the incidence of MACCE at 1 year in patients with multi-vessel coronary disease and DES implantation was 14.4%.15) Another study reported the incidence of MACCE at 1 year to be 12.0% in diabetic patients and 10.4% in non-diabetic patients.16) In the current study, all of the patients that underwent DES implantation during this study period, regardless of the presence of high-risk complex coronary lesions (multi-vessel, calcified coronary disease and diabetes mellitus), were included. The incidence of MACCE at 1 year was shown to be 7.1% in the Plavix® group and 8.0% in the Platless® group, both groups showed relatively low incidence compared with previous reports. During a 1-year follow-up, stroke occurred in 2 patients (0.9%) each in the Plavix® and Platless® groups. Of the MACCE, there was one case of cerebral hemorrhage in the Plavix® group, but there were no hemorrhagic events in the Platless® group. Also, the incidence of major and minor bleeding events was similar between the two groups. This result supports the safety of Platless® with respect to bleeding.
Although there was a statistical difference in the total stent length in the 2 groups (24.04±5.66 in the Plavix® group vs. 22.68±5.94 mm in the Platless® group, p=0.001), our study showed a similar incidence of ST between the 2 groups. The stent length was reported to be an independent predictor of ST, but the threshold of stent length for predicting ST has been reported to be 31.5 mm, which has a sensitivity and specificity of 88.4% and 52.1%, respectively, in a study evaluating the association between the length of the stented segment and the risk of ST.17) In another study evaluating the effect of the stented length and clinical outcomes comparing DESs with bare-metal stents (BMSs), there were higher rates of ST for BMSs in the longest stented length tertile, but there were no significant differences among the different stented length tertiles for DESs.18) Although stent length was significantly different between the two groups in the current study, the difference did not affect the incidence of ST in the Plavix® group. However, the incidence of ST was very low, as previously reported.
The present study has some limitations. First, this is a retrospective observational study which has some known limitations. Differences in baseline characteristics like the incidence of previous PCIs, stent length and medications were not controlled. Also, the present analysis was not conducted at a contemporary period, which may result in differences in stent type (data not shown) and medications. In addition, the sample size of the study group was relatively small and the duration of the follow-up of the study population was relatively short. We excluded the patients that did not complete 1 year of clinical follow-up, which may have affected the relatively low MACCE rates in our study population compared to previous studies. In addition, we did not confirm platelet function using methods such as light transmittance aggregometry or vasodilator-stimulated phosphoprotein, or a platelet function analyzer or point-of-care Verify-Now system. The different response to the anti-platelet effect of clopidogrel can affect clinical outcome, such as MACCEs.19-21) Also in our study, CCBs were prescribed more frequently in the Plavix® group than in the Platless® group and dihydropyridine CCBs were known to inhibit the cytochrome P450 3A4 enzyme, which metabolizes clopidogrel to its active form.22) Thereby, CCBs may lead to an attenuation of clopidogrel-mediated platelet inhibition which may affect the MACCE rate. This reduction of the antiplatelet effect of clopidogrel in patients with concomitant CCB therapy was confirmed by the vasodilator-stimulated phosphoprotein phosphorylation assay23) and light transmission aggregometry and VerifyNow system24) which were not performed in this study. Finally, because the MACCE and ST rates were low, type II error may exist. Particularly, ST occurred in only 1 patient (0.5%) in the Platless® group (vs. 0% in the Plavix® group). Because of the low frequency of ST, adequately powered randomized clinical trials to conclude comparable rate of ST as a secondary endpoint is necessary.
In conclusions, in this study, the 2 tablet preparations of clopidogrel showed similar incidence of MACCE. This study supports generic clopidogrel, Platless®, can be used in patients with DES implantation with a lower cost. However, additional large-scaled, prospective randomized studies comparing Platless® and Plavix® with pharmacodynamics and platelet reactivity are needed to conclude that the generic clopidogrel Platless® could replace the original clopidogrel.
Figures and Tables
 | Fig. 1Diagram showing the number of screened, enrolled, and excluded patients in the study. DES: drug-eluting stent. |
 | Fig. 2The incidence of in-hospital MACCE between the 2 treatment groups (p=0.62). MI: myocardial infarction, TVR: target vessel revascularization, MACCE: major adverse cardiovascular and cerebrovascular event. |
 | Fig. 3The incidence of 1-year MACCEs between the 2 treatment groups (p=0.66). MI: myocardial infarction, TVR: target vessel revascularization, MACCEs: major adverse cardiovascular and cerebrovascular events. |
Table 1
Baseline characteristics of the patients in this study
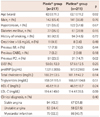
Values are presented as the number (%) or mean±SD. MI: myocardial infarction, CABG: coronary artery bypass graft, PCI: percutaneous coronary intervention, LVEF: left ventricular ejection fraction, proBNP: pro B-type natriuretic peptide, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol
References
1. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001. 345:494–502.
2. Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001. 358:527–533.
3. Steinhubl SR, Berger PB, Mann JT 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002. 288:2411–2420.
4. Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006. 113:2363–2372.
5. Wood S. HeartWire: mixed thoughts on how generic clopidogrel might impact patients, providers [Internet]. 2006. cited 2010 Mar 15. Montreal: theheart.org;Available from: http://www.theheart.org/article/729929.do.
6. Gomez Y, Adams E, Hoogmartens J. Analysis of purity in 19 drug product tablets containing clopidogrel: 18 copies versus the original brand. J Pharm Biomed Anal. 2004. 34:341–348.
7. Kim SD, Kang W, Lee HW, et al. Bioequivalence and tolerability of two clopidogrel salt preparations, besylate and bisulfate: a randomized, open-label, crossover study in healthy Korean male subjects. Clin Ther. 2009. 31:793–803.
8. Bahrami G, Mohammadi B, Sisakhtnezhad S. High-performance liquid chromatographic determination of inactive carboxylic acid metabolite of clopidogrel in human serum: application to a bioequivalence study. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. 864:168–172.
9. Colombo A, Orlic D, Stankovic G, et al. Preliminary observations regarding angiographic pattern of restenosis after rapamycin-eluting stent implantation. Circulation. 2003. 107:2178–2180.
10. Applegate RJ, Grabarczyk MA, Little WC, et al. Vascular closure devices in patients treated with anticoagulation and IIb/IIIa receptor inhibitors during percutaneous revascularization. J Am Coll Cardiol. 2002. 40:78–83.
11. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007. 115:2344–2351.
12. Di Girolamo G, Czerniuk P, Bertuola R, Keller GA. Bioequivalence of two tablet formulations of clopidogrel in healthy Argentinian volunteers: a single-dose, randomized-sequence, open-label crossover study. Clin Ther. 2010. 32:161–170.
13. Jeong YH, Koh JS, Kang MK, et al. The impact of generic clopidogrel bisulfate on platelet inhibition in patients with coronary artery stents: results of the ACCEL-GENERIC study. Korean J Intern Med. 2010. 25:154–161.
14. Robinson A, Hillis J, Neal C, Leary AC. The validation of a bioanalytical method for the determination of clopidogrel in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. 848:344–354.
15. From AM, Al Badarin FJ, Cha SS, Rihal CS. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass surgery for multivessel coronary artery disease: a meta-analysis of data from the ARTS II, CARDia, ERACI III, and SYNTAX studies and systematic review of observational data. EuroIntervention. 2010. 6:269–276.
16. Akin I, Bufe A, Schneider S, et al. Clinical outcomes in diabetic and non-diabetic patients with drug-eluting stents: results from the first phase of the prospective multicenter German DES.DE registry. Clin Res Cardiol. 2010. 99:393–400.
17. Suh J, Park DW, Lee JY, et al. The relationship and threshold of stent length with regard to risk of stent thrombosis after drug-eluting stent implantation. JACC Cardiovasc Interv. 2010. 3:383–389.
18. Applegate RJ, Sacrinty MT, Kutcher MA, Santos RM, Gandhi SK, Little WC. Effect of length and diameter of drug-eluting stents versus bare-metal stents on late outcomes. Circ Cardiovasc Interv. 2009. 2:35–42.
19. Weerakkody GJ, Brandt JT, Payne CD, Jakubowski JA, Naganuma H, Winters KJ. Clopidogrel poor responders: an objective definition based on Bayesian classification. Platelets. 2007. 18:428–435.
20. Barragan P, Bouvier JL, Roquebert PO, et al. Resistance to thienopyridines: clinical detection of coronary stent thrombosis by monitoring of vasodilator-stimulated phosphoprotein phosphorylation. Catheter Cardiovasc Interv. 2003. 59:295–302.
21. Müller I, Besta F, Schulz C, Massberg S, Schönig A, Gawaz M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost. 2003. 89:783–787.
22. Katoh M, Nakajima M, Shimada N, Yamazaki H, Yokoi T. Inhibition of human cytochrome P450 enzymes by 1,4-dihydropyridine calcium antagonists: prediction of in vivo drug-drug interactions. Eur J Clin Pharmacol. 2000. 55:843–852.
23. Siller-Matula JM, Lang I, Christ G, Jilma B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J Am Coll Cardiol. 2008. 52:1557–1563.
24. Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Calcium-channel blockers decrease clopidogrel-mediated platelet inhibition. Heart. 2010. 96:186–189.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download