Abstract
Background and Objectives
The aim of this study was to confirm the predictive cut-off values for P2Y12 reaction units (PRU) and aspirin reaction units (ARU) and to evaluate the clinical impact of VerifyNow® assays.
Subjects and Methods
From November 2007 to October 2009, 186 eligible patients were prospectively recruited. Post-treatment platelet reactivity was measured by VerifyNow® assays within 12 to 24 hours after intervention, followed by standard dual maintenance dose therapy for 1 year. All patients had scheduled clinical follow-ups at 1, 3, 6, and 12 months.
Results
The rate of low responders to clopidogrel, aspirin, and both drugs were 41.4%, 10.2%, and 3.8%, respectively. The predictive factors for low responsiveness to clopidogrel (PRU ≥240) were female sex, age, and non-use of cilostazol medication in our univariate analysis and age ≥65 years and non-use cilostazol in the multivariate analysis. The predictors of low responsiveness to aspirin (ARU ≥550) were male sex and age in both univariate and multivariate analyses. There was no significant difference in the clinical event rate with a cut-off value of PRU ≥240 or ARU ≥550 for 30 days and 1-year (p>0.05).
Conclusion
Hyporesponsiveness to antiplatelet agents (namely aspirin and clopidogrel) was identified in about half of the patients. The cut-off point of PRU ≥240 or ARU ≥550 did not confer predictive value for 30-day or 1-year clinical event rates in patients who had undergone coronary intervention with drug-eluting stents.
The combined use of aspirin and clopidogrel is the cornerstone for patients undergoing percutaneous coronary intervention (PCI) with implantation of drug-eluting stents to prevent short- and long-term major adverse cardiac events, particularly stent thrombosis. However, the combined strategy commonly advocated by guidelines is often complicated by high post-treatment platelet reactivity (HPR) due to inter-individual variability in response to these drugs. The major limitation of low responses to aspirin and clopidogrel is a result of suboptimal antiplatelet effects reported in 5% to 45% of patients treated with aspirin and 4% to 30% of patients treated with clopidogrel. The low responses to these drugs is reported to be associated with the occurrence of clinical adverse events.1-3)
To overcome this problem, three options are now recommended: increasing the dosage of these drugs, adding an additional agent (e.g., glycoprotein IIb/IIIa inhibitor, cilostazol), and switching to more potent antiplatelet drugs (e.g., prasugrel, ticagrelor).4)5) Low responsiveness is also associated with recurrent excessive bleeding,6)7) which has raised great interest in the assessment of platelet reactivity and genetic polymorphisms, with the end goal of possibly tailoring therapy to avoid a "one size fits all" strategy using platelet-function or pharmacogenomic tests. Despite the numerous platelet-function tests that are currently under investigation, none have been specifically recommended for repetitive practice.8)9)
Among these tests, VerifyNow® (Accumetrics Inc., San Diego, CA, USA), a genuine point-of-care assay, has been described as having advantages such as simplicity, speed, user-friendliness, no need for pipetting, and high reproducibility. The aim of this study was to determine the prevalence of hyporesponsiveness with a cut-off value of ≥240 P2Y12 reaction units (PRU) or ≥550 aspirin reaction units (ARU).10)11) We also sought to elucidate the clinical impacts of VerifyNow® monitoring in predicting clinical outcomes in patients undergoing PCI with drug-eluting stent implantation.
Subjects were prospectively recruited as consecutive patients with coronary artery disease who had undergone PCI with drug-eluting stent implantation in the Dong-A University Medical center from November 2007 to October 2009. Patients were considered eligible according to the following criteria: 1) >18 years of age with acute coronary syndrome or new onset of ST-elevation myocardial infarction (MI) over 12 hours or stable angina pectoris not controlled by optimal medical treatment and with at least 70% stenosis of at least one large epicardial coronary artery confirmed by angiography; 2) received a dual loading-dose therapy of 300 mg aspirin (Astrix®, BoRyung Pharm., Korea) and 300 mg clopidogrel (Plavix®, Bristol-Myers Squibb/Sanofi Aventis Pharm., Bridgewater) at least 6 hours before PCI; and 3) underwent platelet function measurements within 12 to 24 hours post-PCI. The exclusion criteria were: 1) >80 years of age; 2) failure to meet aspirin or clopidogrel requirement of loading dose and time; 3) de novo onset of ST-elevation MI within 12 hours; 4) use of a glycoprotein IIb/IIIa inhibitor during PCI procedure; 5) previous PCI or coronary artery bypass surgery within the prior 6 months; 6) ischemic stroke within the prior 6 months; 7) severe renal failure (serum creatinine >2.5 mg/dL); 8) active internal bleeding or thrombocytopenia (platelet count <80000 per liter); 9) allergy to aspirin and/or clopidogrel; 10) planned elective cardiac or non-cardiac surgery in the next 6 months post-PCI; 11) requirement for oral anticoagulation; 12) left ejection fraction of <40%; and 13) treated with any investigational drug within 2 months prior to screening. The study protocol was approved by the institutional review board, and all patients provided a written informed consent for participation.
All eligible patients had been implanted with at least one drug-eluting stent after dual loading-dose therapy of aspirin and clopidogrel as described above. We then performed platelet function measurement by VerifyNow® assays within 12 to 24 hours post-PCI, followed by standard maintenance dose therapy of 100 mg aspirin daily and 75 mg clopidogrel daily for 1 year. All patients had scheduled clinical follow-ups at 1, 3, 6, and 12 months (Fig. 1).
After discarding 3 mL of the initial whole blood to reduce spontaneous platelet activation, blood samples were placed in 2 mL Gr-einer partial fill Vacuette® tubes with 3.2% sodium citrate (Greiner Bio-One, Monroe, NC, USA). Then VerifyNow® Aspirin and VerifyNow® P2Y12 assays were undertaken immediately for HPR. Results of platelet responses to aspirin and clopidogrel were expressed as ARU and PRU.
The primary end points were a composite of major adverse cardiac and cerebrovascular events (MACCE: cardiac death, nonfatal MI, definite/probable stent thrombosis and stroke) at 30 days in terms of the presence or absence of High on-treatment Platelet Reactivity (HPR=low or hypo-responder). The secondary end point was an estimation of the rate of HPR at the post intervention periods in the Korean population after aspirin and clopidogrel administration. Additionally, we also analyzed a composite of MACCE at 1 year.
Definite stent thrombosis was defined as acute coronary syndrome with either angiography confirmation or pathological confirmation of thrombosis. Probable stent thrombosis was defined as unexplained death or MI in the territory supplied by a stented vessel without angiographic confirmation.
Previous studies demonstrated that HPR represents an approximately 3 times greater risk for repeat ischemic events within 30 days of coronary intervention.12) Additionally, using data arising from an Antiplatelet therapy for Reduction of MYocardial Damage during An-gioplasty-Platelet Reactivity Predicts Outcome study,13) we hypothesized that the probability of ischemic events in patients with or without HPR in the current study would be approximately 20% and 6%, respectively.
The estimated sample size required for 80% power with an α of 0.05 is approximately 178 patients. With an anticipated dropout rate of 10%, a total of 198 patients was required.
Continuous variables are presented as mean (standard deviation), and categorical variables are reported as frequencies and percentages. Continuous variables were compared using Student's t-test or Mann-Whitney U test, as appropriate. Categorical variables were compared by χ2 test or Fisher's exact test, as appropriate. A receiver operating characteristic (ROC) curve analysis was used to determine the ability of the VerifyNow® P2Y12 assay to distinguish between patients with or without postdischarge events after PCI.
The optimal cut-off values of PRU for low clopidogrel responders and ARU for low aspirin responders were taken as the cut-off values previously reported in the studies by Marcucci et al.10) (PRU ≥240) and Gum et al.11) (ARU ≥550), which are now widely considered to be optimal cut-off values. A dual low responder was defined as PRU ≥240 and ARU ≥550. Cumulative survival curves for patients with and without low responsiveness were constructed by the Kaplan-Meier method, and the log-rank test was used to assess statistical differences between both patient groups. After assessment of the proportional hazard assumption, univariate and multivariate hazard regression models of Cox were used. The multivariate stepwise forward logistic regression models included all variables (demographic, clinical, and angiographic) that had shown an association with MACCE (a probability p of ≤0.20). A p of <0.05 was considered statistically significant. All statistical analyses were performed with Statistical Package for the Social Sciences (SPSS) 14.0 (SPSS Inc., Chicago, IL, USA).
Out of 200 consecutive patients, a total of 186 patients were eligible as participants in the study from November 2007 to October 2009, with 7 patients refusing to participate, 2 patients missing the check-in time for the platelet function tests, and 5 patients withdrawn due to poor compliance (Fig. 1). Baseline characteristics regarding platelet response to clopidogrel are depicted in Table 1.
Statistical distributions of PPR results in overall groups are shown in Fig. 2. The median (range) of PRU was 222 (10, 453), and the upper quartile was 284. The median (range) of ARU was 406 (92, 634), and the upper quartile was 495. The rate of low clopidogrel responders defined as PRU ≥240 was 41.4%, the rate of low aspirin responders defined as ARU ≥550 was 10.2%, and the rate of the dual low responders defined as ARU ≥550 and PRU ≥240 was 3.8%. The predictive factors for low responders to clopidogrel were female sex, age, and cilostazol non-medication in a univariate analysis and age ≥65 years and non-use cilostazol in multivariate analysis (Table 2). The predictive factors for low responders to aspirin were age and male sex in both univariate and multivariate analysis.
There was significant correlation between PRU and ARU values (r=0.322, p<0.001) and moderate concordance rates in defining low responsiveness (the concordance rate=56%). However, there was no significant agreement between the two values (k=0.013, p=0.79). The plotted relationship is shown in Fig. 3.
Out of 193 patients, a total of 186 patients (96.4%) completed cli-nical follow-up over 12 months (Table 3). The ROC curve analysis de-monstrated that PRU and ARU values have a lower ability to discriminate between patients with and without 30-MACCE {PRU: area under the curve (AUC)=0.51, 95% confidence interval (CI) 0.43 to 0.58, p=0.94; ARU: AUC=0.53, 95% CI 0.45 to 0.60, p=0.69} (Fig. 4).
The cumulative event-free survival curve for 1-year MACCE between the patients with normal responsiveness and low responsiveness was not statistically different (p=0.99). One-year MACCE was also compared between the low responder group to either aspirin or clopidogrel and the normal responder group. There was no statistical difference in event-free survival (p=0.74) (Fig. 5).
Our study showed that there was a higher frequency of clopidogrel hyporesponsiveness (41.4%) than aspirin hyporesponsiveness (10.2%). Dual hyporesponsiveness was only at 3.8%. The PRU and ARU showed significant correlation with a moderate concordance rate. We previously reported the frequency of aspirin and clopidogrel resistance using a light transmittance aggregometer (LTA), VerifyNow® assay, and multiplate electrode analyzer (MEA) assay.14) The prevalence of clopidogrel hyporesponsiveness determined by the VerifyNow® assay was higher than that by the other methods but lower than that by the vasodilator-stimulated phosphoprotein (VASP) phosphorylation assay. In our data, about half of the patients who took both aspirin and clopidogrel had either aspirin, clopidogrel, or dual drugs hyporesponsiveness.
Predictors of clopidogrel hyporesponsiveness have been reported to be associated with gender, age, body mass index, diabetes mellitus, etc.15) In the multivariate analysis of our data, age ≥65 years and non-use of cilostazol were associated with hyporesponsiveness. An association between advanced age and hyporesponsiveness has been identified in many publications,8)16-18) reflecting variables such as decreased renal function and other comorbidities. Therefore, it may be seen as a surrogate marker for low responsive-ness. Female gender has been linked to hyporesponsiveness to clo-pidogrel in some reports,9)19) but male gender has also been linked with hyporesponsiveness to clopidogrel by LTA.20) In our report, female gender was related to clopidogrel hyporesponsiveness, but male gender was related to aspirin hyporesponsiveness. This gender issue is interesting and needs to be further investigated in a large well-designed study.
The cut-off values of clopidogrel hyporesponsiveness by a recent consensus report11) was suggested as follows: 1) platelet reactivity index >50% by VASP phosphorylation assay; 2) PRU >235-240 by VerifyNow® assay; 3) 5-µM adenosine-diphosphate (ADP)-induced maximal aggregation >46% by LTA; and 4) ADP test AUC >468 U by MEA assay. Of these criteria, the PRU value (235-240) derived by MACCE or the upper quartile is much lower than our data (the upper quartile of PRU=284). Also, other cut-off values of PRU reported from other Korean studies are presented as 252.5 and 274.21)22) This discrepancy is reported to be partially related to genetic background differences of the CYP2C19 gene in comparison to Western countries. In order to understand the impact of racial difference and optimal cut-off values in Asian people, a large scale study is needed.
This study did not uncover any association between the predictive cut-off value of PRU ≥240 and increased clinical adverse outcomes, revealing no differences in the prevalence of 1-year MACCE between normal responders and low responders. In the ROC curve analysis, the VerifyNow® assay also did not show a better ability to distinguish between patients with or with MACCE. Put simply, the PPR by the VerifyNow® assay in our patients did not provide any helpful information to predict clinical adverse outcomes.
The recent Gauging Responsiveness with A VerifyNow assay-Impact on Thrombosis And Safety (GRAVITAS) randomized trial23) is a study of tailored antiplatelet therapy regarding HPR that was assessed by the VerifyNow® assay at 12 to 24 hours after PCI; it did not show clinical efficacy of VerifyNow® to discriminate between patients with or without clopidogrel hyporesponsiveness for predicting the occurrence of clinical adverse events. Even though numerous previous studies18)24)25) have reported that HPR is associated with clinical adverse outcomes, and there has been a recent consensus report,11) there is still debate on platelet function testing methods, cut-off points, and loading doses and times. The current laboratory methods of platelet function tests have major limitation, because they are not suitable for repetitive measurements at the bedside.
There are a few studies regarding prediction of cardiovascular events using dual point of care (POC) methods (ARU and PRU). Pinto Slottow et al.26) reported that ARU and PRU values are significantly different between stent thrombosis patients when compared to controls. Also, Lee et al.27) reported the relationship between on-treatment platelet reactivity and a 6-month cardiac event rate. In this report, tertiles of ARU (406, 463) or PRU (184, 265) values were not able to discriminate patients with future thrombotic events, but combining the tertiles of these two values were significantly effective for predicting future events. Interestingly, lower values derived when combining these two values (ARU <406 and PRU <184) did show any ischemic events. Although the GRAVITAS trial did not show the value of a POC device guided with increasing doses of clopidogrel, the role of this device needs to be further evaluated. This is particularly needed in the current era of new antiplatelet agents (e.g., prasugrel, ticagrelor) for the prediction of ischemic events as well as bleeding events.
This prospective observation study had several limitations. First, the small sample size may have been insufficient to uncover relatively rare clinical events, especially stent thrombosis. However, a large scale trial would also have the same problem due in part to improvement of drug-eluting stents structure and emerging PCI techniques and devices. Second, the use of 300 mg of clopidogrel as a loading dose could have a limitation to assess true HPR compared to the higher dose of 600 mg. However, the 300 mg dose followed the guideline recommendations at the start of this study, and we gave the loading dose of 300 mg at least 6 hours before PCI and checked platelet function at least 12 hours later. Therefore, the results of HPR were reliable, because the patients were already in the steady-state. Third, we did not compare other platelet function tests or genetic tests to glean more information about the complexities of platelet hyporesponsiveness.
In conclusion, hyporesponsiveness to antiplatelet agents (namely aspirin and clopidogrel) was identified in about half of the patients. The cut-off points of PRU ≥240 or ARU >550 did not confer predictive value for 30-day or 1-year clinical event rates in patients who had undergone PCI with drug-eluting stent implantation.
Figures and Tables
Fig. 1
Study flow diagram. PCI: percutaneous coronary intervention, MACCE: major adverse cardiac and cerebrovascular events.
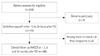
Fig. 2
Statistical distributions of the study patients by VerifyNow® assays. A: P2Y12 reaction units (PRU). B: aspirin reaction units (ARU).
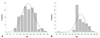
Fig. 3
Correlation, concordance rate, and distribution plot of 1-year MACCE in regards to ARU and PRU values. ARU: aspirin reaction units, MACCE: major adverse cardiac and cerebrovascular events, PRU: P2Y12 reaction units.

Fig. 4
Receiver operating characteristic curve analysis of 30 day MACCE. PRU and ARU values did not enable the ability to predict 30 day MACCE. ARU: aspirin reaction units, MACCE: major adverse cardiac and cerebrovascular events, PRU: P2Y12 reaction units.

Fig. 5
One-year MACCE curve for responders and low responders. There was no significant difference in PRU <240 vs. PRU ≥240 (upper) or PRU ≥240 or ARU ≥550 vs. PRU <240 and ARU <550 (lower). ARU: aspirin reaction units, MACCE: major adverse cardiac and cerebrovascular events, PRU: P2Y12 reaction units.

Table 1
Baseline demographic and clinical characteristics

*PRU ≥240, †Responder vs. low responder. ACE: angiotensin-converting enzyme, BMI: body mass index, LAD: left anterior descending, LCx: left circumflex, LMCA: left main coronary artery, NSTEMI: non ST-elevation myocardial infarction, MI: myocardial infarction, Pre: previous history, PCI: percutaneous coronary intervention, RCA: right coronary artery, STEMI: ST-elevation myocardial infarction, VD: vessel disease, WBC: white blood cells
Acknowledgments
This study was financially supported by grants from the Research Foundation of The Korean Society of Cardiology (2007), and a part of this study was presented as an abstract at The 54th Annual Scientific Meeting of The Korean Society of Cardiology in 2010.
References
1. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007. 49:1505–1516.
2. Angiolillo DJ. Variability in responsiveness to oral antiplatelet therapy. Am J Cardiol. 2009. 103:3 Suppl. 27A–34A.
3. Ferreiro JL, Angiolillo DJ. Clopidogrel response variability: current status and future directions. Thromb Haemost. 2009. 102:7–14.
4. Angiolillo DJ. Platelet function testing in clinical practice: are we ready for prime time? Rev Esp Cardiol. 2009. 62:113–116.
5. Capodanno D, Angiolillo DJ. Platelet monitoring for PCI: which test is the one to choose? Hamostaseologie. 2009. 29:376–380.
6. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007. 357:2001–2015.
7. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009. 361:1045–1057.
8. Tantry US, Gurbel PA. Platelet monitoring for PCI: is it really necessary? Hamostaseologie. 2009. 29:368–375.
9. Campo G, Fileti L, Valgimigli M, et al. Poor response to clopidogrel: current and future options for its management. J Thromb Thrombolysis. 2010. 30:319–331.
10. Marcucci R, Gori AM, Paniccia R, et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. 2009. 119:237–242.
11. Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001. 88:230–235.
12. Aradi D, Komócsi A, Vorobcsuk A, et al. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: systematic review and meta-analysis. Am Heart J. 2010. 160:543–551.
13. Patti G, Nusca A, Mangiacapra F, Gatto L, D'Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) Study. J Am Coll Cardiol. 2008. 52:1128–1133.
14. Woo KS, Kim BR, Kim JE, et al. Determination of the prevalence of aspirin and clopidogrel resistances in patients with coronary artery disease by using various platelet-function tests. Korean J Lab Med. 2010. 30:460–468.
15. Gremmel T, Panzer S. Clinical, genetic and confounding factors determine the dynamics of the in vitro response/non response to clopidogrel. Thromb Haemost. 2011. 106:211–218.
16. Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Adenosine diphosphate-inducible platelet reactivity shows a pronounced age dependency in the initial phase of antiplatelet therapy with clopidogrel. J Thromb Haemost. 2010. 8:37–42.
17. Geisler T, Grass D, Bigalke B, et al. The residual platelet aggregation after deployment of intracoronary stent (PREDICT) score. J Thromb Haemost. 2008. 6:54–61.
18. Price MJ, Endemann S, Gollapudi RR, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008. 29:992–1000.
19. Price MJ, Nayak KR, Barker CM, Kandzari DE, Teirstein PS. Predictors of heightened platelet reactivity despite dual-antiplatelet therapy in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2009. 103:1339–1343.
20. Hochholzer W, Trenk D, Bestehorn HP, et al. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol. 2006. 48:1742–1750.
21. Suh JW, Lee SP, Park KW, et al. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J Am Coll Cardiol. 2011. 57:280–289.
22. Ko YG, Suh JW, Kim BH, et al. Comparison of 2 point-of-care platelet function tests, VerifyNow Assay and Multiple Electrode Platelet Aggregometry, for predicting early clinical outcomes in patients undergoing percutaneous coronary intervention. Am Heart J. 2011. 161:383–390.
23. Price MJ, Berger PB, Teirstein PS, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011. 305:1097–1105.
24. Bliden KP, DiChiara J, Tantry US, Bassi AK, Chaganti SK, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007. 49:657–666.
25. Geisler T, Langer H, Wydymus M, et al. Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. Eur Heart J. 2006. 27:2420–2425.
26. Pinto Slottow TL, Bonello L, Gavini R, et al. Prevalence of aspirin and clopidogrel resistance among patients with and without drug-eluting stent thrombosis. Am J Cardiol. 2009. 104:525–530.
27. Lee SP, Park KW, Shin DH, et al. Efficacy of predicting thrombotic events with combination of dual point-of-care testing (POCT) after drug eluting stent implantation for coronary heart study: result from the CILON-T randomized trial POCT substudy. J Atheroscler Thromb. 2011. 18:914–923.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download