Abstract
One of the single anomalous origins of coronary artery that has rarely been reported is a congenital anomaly of coronary circulation that occurs in the left coronary artery originating from the right coronary sinus of valsalva. We report a 49-year-old male patient with non-ST segment elevated myocardial infarction that was identified to have an anomalous origin of the left coronary artery from the right coronary artery (RCA) with thrombotic total occlusion of RCA by coronary angiography and cardiac computed tomography. The patient underwent successful percutaneous coronary intervention in total occlusion of the RCA and was discharged after uneventful recovery.
Single anomalous origin of coronary artery is a rare congenital anomaly of coronary circulation.1) The incidence of single anomalous origin is approximately 1% among patients undergoing cardiac catheterization, and an anomalous origin of the left coronary artery is particularly less frequent than the right coronary artery (RCA).2-7) We report on a rare case of anomalous left coronary artery that originated from the right sinus of valsalva, with total occlusion on the proximal RCA.
A 49-year-old male suffered from chest pain, dyspnea as well as hypotension and pulmonary congestion 10 hours prior to arrival at the emergency room. Risk factors included smoking and dyslipidemia. The electrocardiogram (ECG) showed ST-segment elevation in aVR and V 1-2, and ST-segment depression in II, III, aVF and V 4-6 (Fig. 1), and troponin-I was elevated (1.35 ng/mL). Under the diagnosis of non-ST segment elevation myocardial infarction, the patient was immediately sent to the catheterization laboratory.
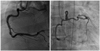
The first time, a coronary angiogram (CAG) suspected an anomalous origin of left coronary arteries and suspected right ventricular branch communicating between proximal RCA and middle left anterior descending coronary artery (LAD) (Fig. 2A), and revealed thrombotic total occlusion in proximal RCA (Fig. 2B). Therefore, for RCA lesion, multiple stepwise plain old balloon angioplasties (POBA) with 1.5×13 mm and 2.5×15 mm balloons, and multiple thrombi suctions with Thrombuster® (Kaneka Corporation, Osaka, Japan) were performed. During the procedure, a temporary pacemaker was inserted due to junctional bradycardia and deepening of mental status. Although multiple thrombi were extracted, huge thrombi remained in distal RCA. Therefore, additional POBA and thrombosuction were performed repeatedly, and intracoronary glycoprotein IIb/IIIa receptor blocker (abciximab) was injected in RCA, after which a 4.0×25 mm bare-metal stent (Coroflex blue®, Braun, Berlin, Germany) was deployed in critical fixed stenosis of the proximal RCA. The RCA showed good distal flow and markedly decreased residual stenosis (Fig. 2C).
The echocardiography noted mild left ventricular systolic dysfunction (EF=43.9% by biplane method), and akinesia in RCA and left circumflex artery (LCX) territory. Cardiac computed tomography (CT) scan showed high calcium score (2036.49) and single origin of coronary artery from right coronary sinus of valsalva with communication between RCA and middle LAD via right ventricular branch (Fig. 3), and moderate degree of luminal narrowing with mixed plaque in middle LAD, and hypoplastic LCX. We maintained IV heparin for the resolution of thrombi on admission.
One week later, we performed follow-up CAG using Amplatz right 2 cm curve (AR2) guiding catheter, CAG revealed patent stent in proximal RCA and a more resolved state of thrombi in PDA, as well as improved distal flow in the posterolateral branch (Fig. 4). After uneventful recovery, the patient was discharged with dual antiplatelet therapy (aspirin 100 mg, clopidogrel 75 mg) and has been followed up at the outpatient clinic.
Anomalous origin of coronary arteries is a rare congenital anomaly that was first described in 1948 by White and Edward.1) The prevalence of this anomaly was determined as 0.6% to 1.3% in an angiographic series and 0.3% in autopsy series.2-6) In particular, incidence of a left coronary anomaly that originates from RCA has been reported to be very rare (0.016% incidence).7) In Korea, a few cases of left coronary anomaly were reported, and most of them were left coronary artery that originated from the right coronary sinus.8,10) Unlike the previous cases, the present case showed a single origin of coronary artery from the right coronary sinus of valsalva with communication between RCA and LAD coronary artery via the right ventricular branch.
Manifestations vary according to subtype of anomalous origin from asymptomatic patients to those who present with myocardial ischmia, angina pectoris, arrhythmia, syncope, and also sudden death, in absence of atherosclerosis.11)12) The pathophysiology basis is unclear. The restricted coronary blood flow in this anomaly suggests that the acute takeoff angle, slit-like orifice, and compression of the intramural segment by the aortic valve commissure are considered to narrow the orifice.13)
The correlation between coronary artery disease and coronary anomalies is uncertain.13) Some previous literature data suggested that anomalous origin of the coronary artery could make them more inclined to atherosclerosis because of altered blood flow pattern.14)15) However, the incidence of coronary artery disease was no different compared to normally-originating coronary arteries.16)17)
The screening method used to evaluate anomalous coronary arteries is important, because this anomaly can be associated with ischemic heart disease and sudden cardiac death. Instrumental advances in multi-detector CT (MDCT) have realized the reduction of imaging time, dose of contrast medium, and radiation exposure. MDCT can be used for non-invasive diagnosis of anomalous origin of coronary arteries, and be useful in increasing the success rate of cannulation during CAG.
Since manifestation varies from benign to sudden death depending on situation, multiple treatment options should be considered including medical, interventional, or surgical procedures.18) Recently, a report suggested that an anomalous coronary artery should be followed without intervention and stated that the benefit of excessive exercise limitation is doubtful. If a young, symptomatic patient has significant luminal narrowing on imaging studies, surgical intervention should be considered.13)19)
In this case, we report a patient with anomalous left coronary artery that originated from the right sinus of valsalva accompanied with total occlusion on the proximal RCA, who underwent successful percutaneous coronary intervention.
Figures and Tables
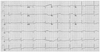 | Fig. 1An electrocardiogram shows ST segment elevation in aVR, and V 1-2, and ST segment depression in II, III, aVF and V 4-6. |
 | Fig. 2Coronary angiogram demonstrated anomalous origin of left coronary arteries and right ventricular branch communicating between proximal right coronary artery and middle left anterior descending coronary artery (A). Right coronary angiogram revealed thrombotic total occlusion (arrow) in proximal right coronary artery (B). After primary percutaneous coronary intervention using multiple thrombosuction and intracoronary stenting, right coronary artery showed good distal flow (C). |
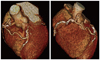 | Fig. 3Cardiac computed tomogram scan demonstrated single origin of coronary artery from right coronary sinus of valsalva with communication between right coronary artery and middle left anterior descending coronary artery via the right ventricular branch. |
 | Fig. 4One week later, we performed follow-up coronary angiogram using Amplatz right 2 cm curve guiding catheter, coronary angiogram revealed patent stent in proximal right coronary artery and more resolved state of thrombi in the posterior descending artery, and improved distal flow in the posterior lateral branch. |
References
1. White NK, Edward JE. Anomalies of the coronary arteries: report of four cases. Arch Pathol (Chic). 1948. 45:766–771.
2. Laureti JM, Singh K, Blankenship J. Anomalous coronary arteries: a familial clustering. Clin Cardiol. 2005. 28:488–490.
3. Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990. 21:28–40.
4. Leberthson RR, Dinsmore RE, Bharati S, et al. Aberrant coronary artery origin from the aorta: diagnosis and clinical significance. Circulation. 1974. 50:774–779.
5. Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation. 1956. 14:800–805.
6. Wilkins CE, Betancourt B, Mathur VS, et al. Coronary artery anomalies: a review of more than 10,000 patients from the Clayton Cardiovascular Laboratories. Tex Heart Inst J. 1988. 15:166–173.
7. Yildiz A, Okcun B, Peker T, Arslan C, Olcay A, Bulent Vatan M. Prevalence of coronary artery anomalies in 12,457 adult patients who underwent coronary angiography. Clin Cardiol. 2010. 33:E60–E64.
8. Cho HO, Cho KH, Jeong YS, et al. Anomalous origin of the left coronary artery from the right sinus of valsalva, which presented as acute myocardial infarction. Korean Circ J. 2006. 36:817–819.
9. Yang DK, Cha KS, Kim BK, et al. Anomalous origin of the left main coronary artery from the right sinus of valsalva. Korean Circ J. 2000. 30:1165–1169.
10. Lee KM, Lee MH, Lee JH, et al. Acute myocardial infarction as a complication of anomalous left coronary artery origin from right coronary sinus. Korean Circ J. 1996. 26:901–905.
11. Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002. 105:2449–2454.
12. Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol. 1992. 20:640–647.
13. Lee BY. Anomalous right coronary artery from the left coronary sinus with an interarterial course: is it really dangerous? Korean Circ J. 2009. 39:175–179.
14. Kimbiris D. Anomalous origin of the left main coronary artery from the right sinus of Valsalva. Am J Cardiol. 1985. 55:765–769.
15. Jim MH, Siu CW, Ho HH, Miu R, Lee SW. Anomalous origin of the right coronary artery from the left coronary sinus is associated with early development of coronary artery disease. J Invasive Cardiol. 2004. 16:466–468.
16. Samarendra P, Kumari S, Hafeez M, Vasavada BC, Sacchi TJ. Anomalous circumflex coronary artery: benign or predisposed to selective atherosclerosis. Angiology. 2001. 52:521–526.
17. Silverman KJ, Bulkley BH, Hulchins GM. Anomalous left circumflex coronary artery: "normal": variant of uncertain clinical and pathologic significance. Am J Cardiol. 1978. 41:1311–1314.
18. Rudan D, Todorovic N, Starcevic B, Raguz M, Bergovec M. Percutaneous coronary intervention of an anomalous right coronary artery originating from the left coronary artery. Wien Klin Wochenschr. 2010. 122:508–510.
19. Gersony WM. Management of anomalous coronary artery from the contralateral coronary sinus. J Am Coll Cardiol. 2007. 50:2083–2084.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download