Abstract
Although it is rare, the right atrium can be encroached on by abnormal mediastinal structures, including aortic aneurysms, carcinomas, hepatic cysts and diaphragmatic paralysis. Extrinsic compression of the right atrium causes significant hemodynamic compromise and can lead to fatal outcomes. We describe the case of a 66-year old man with a past history of pulmonary tuberculosis that had undergone right pneumonectomy 40 years previously. He then presented with signs and symptoms of right-sided heart failure. These new signs and symptoms were recognized to be secondary to extrinsic compression of the right atrium, which was due to late-onset postpneumonectomy empyema, and the signs and symptoms were successfully relieved by performing open drainage of the empyema.
Empyema is an uncommon complication of pneumonectomy and may occur within 1 day or several weeks after pneumonectomy. Although postpneumonectomy empyema (PPE) is usually diagnosed during the same hospital visit, some cases are not detected until months or even several years later. Late-onset PPE (>3 month) may be very difficult to diagnose and manage. Because of the high mortality rate for PPE with combined complications such as bronchopleural fistula, early diagnosis and optimal management is important. We describe the case of a 66-year old man presenting with symptoms and signs of right-sided heart failure due to extrinsic compression of the right atrium caused by late-onset PPE.
A 66-year old man presented with a two week history of shortness of breath, facial edema and edema in both lower extremities. Forty years prior to this presentation, the patient suffered from primary pulmonary tuberculosis of his right lung. He underwent right pneumonectomy and was treated with antituberculosis therapy for 1 year, with no subsequent problems. The current clinical examination revealed evidence of right-sided heart failure, including peripheral edema and shortness of breath at rest.
At admission, the patient's physical examination revealed a blood pressure of 140/90 mm Hg, a pulse rate of 119/minute, a respiration rate of 24/minute and a body temperature of 36℃. The breathing sounds diminished in the right hemithorax and crackles and wheezing sounds were heard on the left lung field. Any abnormal lymph nodes were not palpable, and there was no hepatosplenomegaly. There was pretibial pitting edema on both lower legs.
An evaluation with laboratory tests revealed a hemoglobin concentration of 11.8 g/dL, a white blood cell (WBC) count of 8220 cells/µL (neutrophils: 72%, lymphocytes: 14%), a platelet count of 267000/µL, an increased erythrocyte sedimentation rate of 29 mm/hr, C-reactive protein 7.2 mg/dL and the lactate dehydrogenase (LDH) level 482 IU/L. The results of the HIV enzyme linked immuno sorbent assay were negative.
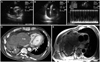
A chest radiograph showed total haziness in the right lung field, and this was probably the result of the past pneumonectomy. Despite conventional management, including oxygen and a bronchodilator, his condition became worse with worsening despnea at rest, weight gain over 5 kg and increased pretibial pitting edema. The transthoracic echocardiogram revealed a large pericardial effusion in the free wall of the right atrium (Fig. 1A and B). Pulsed Doppler echocardiography demonstrated an abnormally increased respiratory variation in transvalvular blood flow velocities (Fig. 1C). Extrinsic right atrial compression was suspected, but was not verified via transthoracic echocardiography due to accompanied severe pericardial effusion. Systolic functions were observed to be within the normal ranges. A diagnostic and therapeutic pericardiocentesis was immediately performed. The pericardial fluid showed a lymphocyte-predominant exudate with an increased protein content (3800 g/dL), an elevated level of LDH (1612 U/L), a depressed glucose level (104 mg/dL), WBC count of 1180/mm3 (neutrophils: 35%, lymphocytes: 65%) and an increased level of adenosine deaminase (ADA) of 53.9 U/L. The pericardial fluid acid fast bacilli (AFB) staining and culture were negative. The polymerase chain reaction we performed for Mycobacterium tuberculosis complex from the pericardial fluid was negative. Cytologic examination demonstrated no malignant cells. During the pericardial drainage, a contrast enhanced chest computed tomography (CT) was performed to evaluate the potential causes of the extrinsic right atrial compression. The chest CT showed that a huge empyema was compressing the right atrium (Fig. 1D). Subsequent magnetic resonance imaging confirmed this result (Fig. 1E).
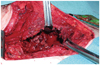
The patient subsequently underwent open drainage of the empyema with an Eloesser flap. The pleural cavity was filled with a large amount of empyema debris and this was completely removed (Fig. 2). After the operation, follow-up Doppler echocardiography showed no respiratory variation of mitral inflow E wave velocity. This finding suggested that AFB and Gram staining of the body fluid from the open drainage, including for anaerobes, remained sterile. The patient recovered with resolution of all his signs and symptoms of right atrial compression after the completion of treatment.
The heart is centrally located in the mediastinum, so it can be encroached on by abnormal structures originating from the anterior, posterior or superior mediastinum. D'Cruz et al.1) divided the relationship between the mediastinal masses and the heart into three categories: 1) proximity: a contiguous or adjacent structure without chamber deformation, 2) encroachment: distortion of the normal cardiovascular architecture without a hemodynamic effect and 3) compression. If the mediastinal mass is large and rigid or if it is cystic and under high pressure and located in a way that it would compress the heart, it can produce hemodynamic effects and symptoms akin to tamponade, as seen in our patient. The compressed right atrium of our patient led to two catastrophic effects: right-sided heart failure and cardiac tamponade. Fowler et al.2) described a similar mechanism that was responsible for causing isolated right atrial tamponade in experimental animals. A negative transmural right atrial pressure due to the right ventricular diastolic suction was observed in that study. In addition, a significant pressure gradient between the superior vena cava and the right atrium, suggesting caval compression during right atrial tamponade, was noted in that study. Vena caval compression was not observed when the animals were subjected to tamponade of the entire heart. Thus, presumably, both the loss of the negative transmural right atrial pressure gradient and the caval compression are responsible for causing hemodynamic effects in isolated right atrial compression.
Empyema that occurs after pneumonectomy is a major complication that is usually diagnosed during the same period of hospitalization. This may occur within 1 day or several weeks after pneumonectomy, and some empyemas occur several months or even several years after a pneumonectomy. The incidence of late-onset (>3 months) PPE seems to be quite low, and only a few cases of late-onset PPE have been reported over the last 25 years.3-8) In our patient, a PPE was diagnosed more than 40 year after his pneumonectomy for tuberculosis. The pathogenesis of late-onset PPE is sometimes difficult to establish because of the many possible sources of infection of the postpneumonectomy space. The hematogenous spread is the usual cause of infections that occur at a later time after surgery. No organism was isolated in 20% of the patients who had frank pus in their pneumonectomy space.9) Likewise, the cause of the empyema in our patient remained uncertain because of the negative results of AFB and Gram staining as well as the culturing, and this included mediums for anaerobic organisms. However, we believed that the presumptive causal organism was tuberculosis from some evidence, including the past history of tuberculosis, as well as increased ADA and lymphocyte-dominant purulent pericardial fluid. We then started a full course of antituberculosis medication.
In general, diagnosing late-onset PPE is difficult because these patients are commonly asymptomatic or have nonspecific symptoms such as anorexia, increased body temperature, weight loss, chest discomfort and/or pain. Our patient did not visit the hospital until his disease manifested as right-sided heart failure. Transthoracic echocardiography is of limited value for diagnosing right atrial compression, but is useful to evaluate hemodynamic changes and cardiac function. Chest CT is the test of choice for identifying masses adjacent to the right atrium. It may be used not only to determine the interrelation between the mass and cardiac structures, but also to identify the etiologic cause of the disease. PPE is considered the most problematic among postoperative pleural infections, and is associated with very high morbidity and a mortality rate of 13-50%.10) Therefore, the optimal management of complicated empyema is based on an early diagnosis, appropriate antibiotic treatment and prompt drainage of the pleural space.
In conclusion, extrinsic compression of the right atrium is a rare cause of heart failure. Other adjacent abnormal mediastinal structures that include an aortic aneurysm, carcinoma, a hepatic cyst and diaphragmatic paralysis may also cause right atrial compression. Our case is a rare and unique cause of right-sided heart failure due to significant right atrial compression, and the latter was caused by late-onset PPE in the right pleural space. Late-onset PPE is very rare and can cause complicated illnesses in postpneumonectomy patients. It is crucial that an early, accurate diagnosis is made and adequate drainage has been done so that further intervention can proceed in a timely manner.
Figures and Tables
Fig. 1
A and B: a short-axis and an apical 4-chamber transthoracic echocardiography view demonstrated a pericardial effusion of over 2 cm around the free wall of the right ventricle. C: respiratory variations of transmitral Doppler inflow: a decrease of peak flow velocity with >25% (from 1.1 to 0.5 m/s) during inspiration. D: post contrast CT at the level of the left ventricle. The large mass with a mean CT number of 40 is empyema. Note the thick pleura with calcifications (arrows), and this represents a fibrothorax from the tuberculosis. The right atrium is markedly compressed by the empyema. A small to moderate amount of left pleural effusion is noted (the mean CT number is 25). E: axial image of chest MRI. A large mass in the right thorax compresses the right heart and vena cava. The mass has a lobular fibrous capsule of high signal intensity and a central low signal intensity. The thick fibrous capsule shows various degrees of enhancement (arrows). FA of 180 degrees, a TR of 976 milliseconds and a TE of 12 milliseconds. PE: pericardial effusion, Emp: empyema, RA: right atrium, RV: right ventricle, LA: left atrium, LV: left ventricle.
References
1. D'Cruz IA, Feghali N, Gross CM. Echocardiographic manifestations of mediastinal masses compressing or encroaching on the heart. Echocardiography. 1994. 11:523–533.
2. Fowler NO, Gabel M, Buncher CR. Cardiac tamponade: a comparison of right versus left heart compression. J Am Coll Cardiol. 1988. 12:187–193.
3. Kerr WF. Late-onset post-pneumonectomy empyema. Thorax. 1977. 32:149–154.
4. Model D. Occult empyema presenting ten years after pneumonectomy. Lancet. 1983. 1:192–193.
5. Rogiers P, Van Mieghem W, Engelaar D, Demedts M. Late-onset post-pneumonectomy empyema manifesting as tracheal stenosis with respiratory failure. Respir Med. 1991. 85:333–335.
6. Stafford EG, Clagett OT. Postpneumonectomy emphema: neomycin instillation and definitive closure. J Thorac Cardiovasc Surg. 1972. 63:771–775.
7. Berge MV, Snoek WJ, Nijboer EW, Julius AJ, Aalbers R. A man with upper respiratory tract infections, general weakness and fever. Eur Respir J. 1999. 14:469–470.
8. Moon JY, Jeong HC, Cho JY, et al. Anomalous origin of a right coronary artery with extrinsic compression between the great vessels: the intravascular ultrasound images. Korean Circ J. 2008. 38:390–392.
9. Goldstraw P. Treatment of postpneumonectomy empyema: the case for fenestration. Thorax. 1979. 34:740–745.
10. Wain JC. Management of late postpneumonectomy empyema and bronchopleural fistula. Chest Surg Clin N Am. 1996. 6:529–541.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download