Abstract
Background and Objectives
Vascular rings refer to anomalies of the great arteries that cause respiratory or feeding problems. The purpose of this study was to analyze a series of patients with vascular rings and evaluate associated risk factors for mortality.
Subjects and Methods
A retrospective review of all patients identified with vascular rings between 1997 and 2010 in the Seoul National University Children's Hospital.
Results
Thirty-five patients were diagnosed with vascular rings (median age at diagnosis, 7 months). The vascular rings of 32 patients were confirmed by cardiac computed tomography or magnetic resonance imaging. The types of vascular rings were: a double aortic arch in ten patients, a right aortic arch with persistent left ligamentum arteriosum in seven, an aberrant subclavian artery in seven, a pulmonary sling in eight, and others types in three patients. Eleven patients were asymptomatic. Gastrointestinal and respiratory symptoms were seen in ten and sixteen patients, respectively. Associated cardiovascular anomalies were present in fifteen patients. Twenty patients with definite symptoms underwent surgical treatment. The median interval between diagnosis and operation was 6 days. Four patients eventually died; three deaths were associated with complex heart diseases, and one had pulmonary artery sling with severe tracheal stenosis. Only the presence of a complex heart disease significantly influenced mortality (p=0.002).
Vascular rings are rare congenital anomalies that primarily result from an embryological derangement of the paired aortic arches or branching pulmonary arteries.1)2) The symptoms and physical findings produced by vascular rings are related to the structure(s) they encircle: the trachea, esophagus, or both. In most centers, cardiac computed tomography (CT) angiography or magnetic resonance imaging (MRI) is used to confirm the diagnosis for surgical planning since the early 2000s, instead of esophagography and angiography.1-5) The diagnosis of vascular rings frequently requires surgical repair according to the individual type of ring, and results in an improvement of symptoms in most cases.6)7) There have not been any studies evaluating the risk factors for mortality from vascular rings, until now. This study was performed to analyze the clinical characteristics and treatments of patients with various vascular rings and evaluate associated risk factors for mortality.
Between 1997 and 2010, we retrospectively reviewed the medical records of all patients with vascular rings at Seoul National University Children's Hospital. Demographic data including: sex, current age, age at diagnosis, presenting symptoms, diagnostic tools, structures compressed by the vascular ring, and associated anomalies were recorded for each patient. All patients were assigned to 5 vascular ring subtypes: 1) double aortic arch, 2) right aortic arch with a persistent left ligamentum arteriosum, 3) aberrant subclavian artery, 4) pulmonary artery sling, and 5) others (Fig. 1).8) Compressed structures were divided into the airway and esophagus. Associated anomalies were divided into cardiac and non-cardiac anomalies. Treatments included operative and non-operative treatments. In cases of surgical treatment, we assessed: age at the time of operation, duration of intubation, duration of intensive care unit (ICU) stay, hospital days, and postoperative complications. We also reviewed mortality cases, including: the interval between diagnosis and operation, cause of death, and other risk factors. Ten variables were examined for their possible influence on mortality: age at presentation, sex, type of vascular ring, complete/incomplete ring, presence of complex heart disease, presence of respiratory anomaly, initial respiratory arrest, airway compression, interval between diagnosis and operation, and ICU stay. Early death was defined as death during hospitalization or within 30 days after surgical repair. This study protocol was approved by the Ethics Committee of our institution, which waived the patient consent because of the retrospective nature of data analysis.
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) Statistics 18.0 (SPSS Inc., Chicago, IL, USA). Data were described as frequencies, medians with ranges, and means with standard deviations as appropriate. Factors associated with mortality were compared using Fisher's exact test for a small population. A p<0.05 was considered statistically significant.
We enrolled 35 patients who had been diagnosed with vascular rings. Twenty-four patients were male and eleven were female. The current age among the patients who survived was 8.4 years (1.5-22.5 years). The average follow-up duration among the enrolled patients was 5.7±5.0 years. The median age at the first onset of symptoms was 78 days (birth to 8.8 years), and median age at diagnosis was 7 months (birth to 8.8 years).
The subtypes of vascular rings and individual clinical profiles are shown in Table 1. A double aortic arch occurred in ten patients, right aortic arch with persistent left ligamentum arteriosum in seven, aberrant right or left subclavian artery (SCA) in seven, pulmonary sling in eight, and others in three patients. These other three types of vascular rings included 1) circumflex retroesophageal arch and aberrant left SCA with ventricular septal defect (VSD), 2) circumflex retroesophageal right aortic arch with coarctation of the aorta, and 3) right aortic arch with mirror-image branching of arch vessels with right isomerism and dextrocardia, severe pulmonary stenosis (PS), complete atrioventricular septal defect (AVSD), and four major aortopulmonary collateral arteries (MAPCA). Arch dominance in the double aortic arch was right-sided in nine patients and balanced in one. The compressed structures were the esophagus in twenty patients (57.1%) and the airway in twenty-one (60%). Among them, the esophagus and airway were both compressed in nine patients (25.7%) and the compressed structures were absent in 3 patient.
Eleven patients (31.4%) were asymptomatic at the time of diagnosis. Seven presented with cyanosis or a murmur due to associated cardiac anomalies and one patient complained of a headache, unrelated to the vascular ring. Four patients with aberrant SCA and three patients with a double aortic arch were asymptomatic. Gastrointestinal symptoms were found in ten (28.6%) and respiratory symptoms in sixteen patients (45.7%). Two patients had both gastrointestinal and respiratory symptoms. The most common gastrointestinal symptom was feeding difficulty, present in ten patients (28.6%), five of which with a right arch and persistent left ligamentum. The most common respiratory symptom was respiratory difficulty, occurring in eleven patients (31.4%), followed by wheezing, recurrent respiratory infection, and coughing. Among them, one patient with tracheal stenosis presented with a failure to wean from mechanical ventilation. Three patients (8.6%) presented with respiratory arrest or apnea. Overall, eleven patients (50%) showed symptoms in the neonatal period and seven (20%) at birth. There was no correlation between age at the onset of symptoms, and the existence of cardiac defects, among different vascular ring types.
Diagnostic modalities are shown in Table 2. The preferred tool for initial evaluation and screening for vascular rings was echocardiography (EchoCG), which indicated vascular rings in fourteen patients (40%). Thirty percent of cases with a double aortic arch were identified by EchoCG, which also proved to be of suggestive value in other diagnoses. Twenty-nine patients (82.9%) were confirmed to have vascular rings by cardiac CT including all patients with an aberrant SCA. Three patients (8.6%) were confirmed by cardiac MRI. Barium esophagography was used in twelve patients for initial evaluation. Bronchoscopy was performed in two of the eight patients with a pulmonary artery sling.
Associated cardiovascular anomalies were present in fifteen patients (42.9%) including a persistent left superior vena cava in eight patients (22.9%), VSD in six (17.6%), and a patent ductus arteriosus in five (14.3%). Of these patients, four had a complex heart disease: tetralogy of Fallot, transposition of great arteries, pulmonary atresia, and severe PS with complete AVSD.
Associated non-cardiac anomalies included respiratory anomalies in 13 patients (40.6%). Among them, nine patients (25.7%) were diagnosed with tracheal stenosis (seven of which were associated with a pulmonary sling), four with tracheomalacia, and one with laryngomalacia. Six patients (17.1%) had genetic abnormalities: CATCH 22 syndrome (four patients), Rubinstein-Taybi syndrome (one), and chromosomal aberration syndrome (one).
Twenty patients (57.1%) underwent surgical repair at a median age of 6.5 months (11 days to 8.9 years). Patients with pulmonary artery sling underwent surgery at a median age of 6.5 months (4 to 36 months) (Table 1). The median interval between diagnosis and surgery was 6 days (0 to 8 months) and between symptoms and surgery 2.1 months (8 days to 4.9 years). In the right aortic arch with persistent left ligamentum group, most patients had relatively mild symptoms, which lead to delayed diagnosis and surgical repair compared to other types (Table 1).
Five asymptomatic patients underwent surgical repair. Among them, four patients underwent surgery because of associated complex cardiac anomalies and one patient, whose brother had moyamoya disease, complained of headache and had blood pressure discrepancies between both arms. He underwent elective surgical repair because thoraco-abdominal CT showed a double aortic arch.
Eight of the ten patients with a double aortic arch were treated surgically. The non-dominant arch was divided in four cases. A dominant right arch with stenotic isthmus was divided in one case and one patient with balanced aortic arch underwent resection of the isthmus of the left aortic arch. In all eight patients, ligamentum arteriosum (5 cases), atretic ligament (1 case) or patent ductus arteriosus (3 cases) were also divided. Three out of seven patients with a right aortic arch with persistent left ligamentum underwent vascular ring repair: division of ligamentum arteriosum and atretic segment in one patient, division of atretic segment in one patient, and division of ligamentum arteriosum in one patient. Six patients with a right aortic arch and left ligamentum and one patient with aberrant SCA had a persistent Kommerell diverticulum. A diverticulectomy was performed in 1 of 6 patients with a right aortic arch with persistent left ligamentum. Two patients with an aberrant SCA underwent surgical repair. One of them underwent left ductal division with concurrent repair of a transposition of great vessels (arterial switch operation). The other patient underwent division of aberrant right SCA and reimplantation to the right common carotid artery. All six patients with pulmonary artery sling underwent division of left pulmonary artery and reimplantation. Ductal division was performed in three patients and division of ligamentum arteriosum was performed in one patient.
Airway surgery including aortopexy of the descending aorta and tracheopexy was performed in three patients (8.6%). Two patients diagnosed with a double aortic arch underwent tracheostomy. The indications for tracheostomy were vocal cord palsy and subglottic edema after vascular ring repair in one patient and tracheomalacia at diagnosis of vascular ring in another patient.
The surgical repair was performed via a median full medial sternotomy in all patients with pulmonary artery sling. Surgical approach in the fourteen patients with the other types included left posterolateral thoracotomy in seven patients median, full medial sternotomy in six and unknown in one. Six patients with concurrent heart disease required a median sternotomy.
Surgical repair was not performed in fifteen patients. Eight patients remained asymptomatic; two of these patients and three others were lost-in-follow-up. Two of the right aortic arch with persistent left ligamentum patients did not undergo surgery, one had mild symptoms and one patient is waiting for surgical repair.
The median hospital stay after surgery was 17 days (6 days to 5.7 months) and ICU stay was 3.5 days (0 to 5.7 months). This prolonged median hospital stay is due to one patient, with a pulmonary sling and long segment tracheal stenosis, who stayed in the ICU for approximately 6 months for ventilator weaning failure.
The median hospital and ICU stays for patients with a pulmonary sling were longer than for other types of vascular rings: 55 vs. 13 days and 26 vs. 3 days, respectively. The median duration of intubation was also longer in patients with pulmonary sling, 20 days (0.56-173 days) versus 9 hours (0.1-16.8 hours) for other types. The duration of postoperative follow-up was 5.28±4.17 years (7 days to 12.5 years). Postoperative complications included: chylothorax (one case); vocal cord palsy (two cases); sepsis (three cases); and pneumothorax (three cases). Among the sixteen patients with respiratory symptoms, eleven underwent surgical repair including airway surgery. Eight of them were cured, one had partial improvement, one patient had persistent symptoms, and the patient with a pulmonary sling and tracheal stenosis died.
For the three patients undergoing airway surgery, postsurgical follow-up exceeded 12 months, with an average of 45 months. Two patients who required aortopexy recovered completely. One patient who required tracheopexy continued to have recurrent dyspnea with wheezing after 95 months of follow-up.
There was no intraoperative mortality, but four patients died (Table 3). One patient with a pulmonary sling and severe long-segment tracheal stenosis died of sepsis and respiratory failure. Two patients (10%) of 20 patients died within 1 month after surgical repair of a complex heart defect: one from sepsis and respiratory failure and the other from shunt malfunction and severe chylothorax. The patient diagnosed with mirror-image branching of the arch vessels and complex heart defects (right isomerism and dextrocardia, complete AVSD, PS, and MAPCAs) was planned to undergo repair after grow-up 2 or 3 months. However, the patient died of sudden cardiac arrest while waiting.
The factors associated with mortality are shown in Table 4. We found that only the presence of complex heart disease significantly influenced mortality. Age at presentation, gender, vascular ring type, presence of respiratory anomalies and airway compression, presence of initial respiratory arrest, interval between diagnosis and surgery, and ICU stay did not appear to be significantly linked with mortality.
Not all the patients with vascular rings needed surgical repair. Among the 35 patients, eleven patients (31.4%) were asymptomatic and only twenty patients (57.1%) showed definite symptoms needing surgical repair. In our study, fewer patients required surgical repair compared to previous studies.6)9)
Vascular ring anomalies causing tracheoesophageal compression comprise 1-3% of all congenital cardiac anomalies.10-12) The reported incidence of respiratory symptoms and signs in patients with vascular rings is 70-95%, and that of gastrointestinal symptoms 5-50%.6)9) Patients with severe compression tend to present symptoms earlier in life. More than half the patients showed symptoms during the neonatal period and respiratory symptoms were more prominent. The most common respiratory and gastrointestinal symptoms were similar to those found in previous studies.3)6)13) Some patients may show symptoms much later in life and some remain asymptomatic throughout their entire life.
The presence of common pediatric signs and symptoms associated with tracheoesophageal compression should alert us to the possibility of a vascular ring. The diagnosis should be made immediately because patients with any type of vascular rings are at risk of life-threatening complications (8.6% of our patients) such as respiratory arrest or apnea.3)7)14)
As shown in previous studies, chest radiography and barium es-ophagography are important for excluding other causes of common respiratory symptoms.1-3)6)7)14)15) In our study, chest radiography was performed in four patients and barium esophagography in twelve patients. But these modalities were of lesser importance to the diagnostic process compared to EchoCG, cardiac CT or MRI.
Echocardiography is a noninvasive, easily available, and important diagnostic modality to accurately assess anatomy and exclude other intracardiac anomalies.3)6)13) It is important to identify associated cardiac anomalies because they are quite frequent and associated with a poor prognosis. However, EchoCG has its limitations for accurately identifying vascular structures in these anomalies.15) The diagnostic process has been aided by cardiac catheterization in the past.3)16) Currently, CT angiography is an important modality used in our center. It accurately defines the type of anomaly in most patients and directly shows the relationship of the arch to the trachea and bronchi.1-5)
In our study, the median interval between diagnosis and surgery was only 6 days and the time between symptoms and surgery was about 2 months. The interval between symptoms and surgery was mainly dependent on the time between symptoms and diagnosis, because we quickly performed surgical repair after confirming the diagnosis. We expected that early repair (surgery within 1 month of age) would lead to improved mortality, yet no correlation was found between early repair and mortality. Three patients died after surgical repair and two of them were asymptomatic at the time of elective operation for complex heart disease. The main causes of death were postoperative complication such as sepsis and respiratory failure.
Overall prognosis for a vascular ring was relatively good in this study, though vascular rings combined with complex heart disease showed a poor prognosis.
In conclusion, vascular rings include several types of anomalies, each with different symptoms and prognosis. The presence of complex heart disease was significantly associated with mortality. Surgical repair for a vascular ring has a relatively good prognosis and the operative risk in the absence of complex heart disease is low. Therefore, early diagnosis and timely surgery in symptomatic patients are paramount. In contrast, patients with mild symptoms can be managed medically with close follow-up.
Figures and Tables
Fig. 1
The three-dimensional structures of diverse types of vascular rings, reconstructed from cardiac computed tomography. A: double aortic arch; two aortic arches (white bold arrows) comprise the complete ring in this patient. B: right aortic arch with persistent left ligamentum arteriosum; Kommerell's diverticulum (arrowhead) and fibrotic band (white dotted arrows) comprise the complete ring in this patient. C: right aortic arch with aberrant left subclavian artery (red bold arrow). D: pulmonary sling; left pulmonary artery originates from right pulmonary artery (red dotted arrow), compressing the bronchus.
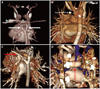
Table 3
Mortality cases

M: male, F: female, SCA: subclavian artery, DAA: double aortic arch, LPA sling: left pulmonary sling, TGA: transposition of great arteries, IVS: intact ventricular septum, ASD: atrial septal defect, TOF: tetralogy of Fallot, PA: pulmonary atresia, VSD: ventricular septal defect, AVSD: atrioventricular septal defect, PS: pulmonary stenosis, MAPCA: major aortopulmonary collateral arteries, Op.: operation, Pt.: patients, Rt.: right
References
1. Kellenberger CJ. Aortic arch malformations. Pediatr Radiol. 2010. 40:876–884.
2. Hernanz-Schulman M. Vascular rings: a practical approach to imaging diagnosis. Pediatr Radiol. 2005. 35:961–979.
3. Ruzmetov M, Vijay P, Rodefeld MD, Turrentine MW, Brown JW. Follow-up of surgical correction of aortic arch anomalies causing tracheoesophageal compression: a 38-year single institution experience. J Pediatr Surg. 2009. 44:1328–1332.
4. Banka P, Geva T, Powell AJ, Geggel R, Lahiri T, Valente AM. Images in cardiovascular medicine: right aortic arch with aberrant left innominate artery: a rare vascular ring. Circulation. 2009. 120:264–265.
5. Eichhorn J, Fink C, Delorme S, Ulmer H. Rings, slings and other vascular abnormalities: ultrafast computed tomography and magnetic resonance angiography in pediatric cardiology. Z Kardiol. 2004. 93:201–208.
6. Humphrey C, Duncan K, Fletcher S. Decade of experience with vascular rings at a single institution. Pediatrics. 2006. 117:e903–e908.
7. Dodge-Khatami A, Tulevski II, Hitchcock JF, de Mol BA, Bennink GB. Vascular rings and pulmonary arterial sling: from respiratory collapse to surgical cure, with emphasis on judicious imaging in the hi-tech era. Cardiol Young. 2002. 12:96–104.
8. Park MK. Fletcher J, McGonigal C, editors. Vascular ring. Pediatric Cardiology for Practitioners. 2008. 5th ed. Philadelphia: Mosby Elsevier;303–306.
9. Binet JP, Longlois J. Aortic arch anomalies in children and infants. J Thorac Cardiovasc Surg. 1977. 73:248.
10. Gross RE. Surgical relief for tracheal obstruction from a vascular ring. N Engl J Med. 1945. 233:586–590.
11. Han BS, Kim CH, Oh BH, et al. A case of right sided aortic arch causing superior vena cava syndrome. Korean Circ J. 1989. 19:776–779.
12. Bonnard A, Auber F, Fourcade L, Marchac V, Emond S, Révillon Y. Vascular ring abnormalities: a retrospective study of 62 cases. J Pediatr Surg. 2003. 38:539–543.
13. Turner A, Gavel G, Coutts J. Vascular rings: presentation, investigation and outcome. Eur J Pediatr. 2005. 164:266–270.
14. Woods RK, Sharp RJ, Holcomb GW 3rd, et al. Vascular anomalies and tracheoesophageal compression: a single institution's 25-year experience. Ann Thorac Surg. 2001. 72:434–438.
15. Alsenaidi K, Gurofsky R, Karamlou T, Williams WG, McCrindle BW. Management and outcomes of double aortic arch in 81 patients. Pediatrics. 2006. 118:e1336–e1341.
16. Kocis KC, Midgley FM, Ruckman RN. Aortic arch complex anomalies: 20-year experience with symptoms, diagnosis, associated cardiac defects, and surgical repair. Pediatr Cardiol. 1997. 18:127–132.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download