Abstract
Background and Objectives
The redox system is an important anti-oxidative system composed of thioredoxin, thioredoxin reductase, and peroxiredoxin (PRx). The fine details of PRx expression and its protective effects in various cells in cardiovascular tissue under oxidative stress created by hydrogen peroxide have not been fully elucidated.
Subjects and Methods
Oxidative stress was induced by adding hydrogen peroxide at 0.25 mM for 2 hours to rat neonatal cardiomyocytes (rCMCs), rat vascular smooth muscle cells (rVSMCs), and human umbilical vein endothelial cells (HUVECs). Apoptosis was quantified by flow cytometry and the expression patterns of the six PRx isoforms were evaluated by western blotting in the three cell lines after hydrogen peroxide stimulation. Apoptosis and the cell survival signal pathway were evaluated by PRx1 gene delivery using lentiviral vector in hydrogen peroxide stimulated rCMCs versus green fluorescence protein gene delivery.
Results
Hydrogen peroxide induced 25% apoptosis in rCMCs. Furthermore, the PRx1 and 5 isoforms were found to be overexpressed in hydrogen peroxide treated rCMCs, and PRx1 overexpression by gene delivery was found to reduce hydrogen peroxide induced rCMCs apoptosis significantly. In addition, this effect was found to originate from cell survival pathway modification.
Conclusion
Hydrogen peroxide induced significant oxidative stress in rCMCs, rVSMCs, and HUVECs, and PRx1 overexpression using a lentiviral vector system significantly reduced hydrogen peroxide induced rCMCs apoptosis by upregulation of cell survival signals and downregulation of apoptotic signals. These findings suggest that PRx1 could be used as a treatment strategy for myocardial salvage in conditions of oxidative stress.
Oxidative stress is caused by an imbalance between the generation of oxidants and antioxidants in the body. Oxidative stress increases the formation of reactive oxygen species (ROS) or reactive nitrogen species.1) ROS contains one or more unpaired electrons in their outer orbits, causing them to be highly reactive.2) These species are generated constantly in vivo, and can cause oxidative damage to nucleic acids, lipids, and proteins, and affect cell membrane properties. Furthermore, their accumulation may lead to the oxidative destruction of cells.3)
Reactive oxygen species also play central roles in cardiac physiology or pathophysiology, and have been shown to injure both endothelial cells and cardiomyocytes (CMCs) via various molecular pathways.4)5) In addition, ROS may be an important cause of atherosclerosis, ventricular hypertrophy and its related cardiomyopathy.5) Coronary atherosclerosis is a direct cause of ischemic heart disease and oxidative stress has been suggested as a cause of atherosclerotic plaque instability and rupture. Thus, they are believed to play an important role in the pathophysiology of acute myocardial infarction (AMI) and its related ventricular remodeling.6) The excessive generation of ROS endogenously has been directly related with metabolic stress, apoptosis, and necrosis in mammalian CMCs.7)8) Ischemia and reperfusion are major causes of oxidative stress in CMCs, showing that oxidative stress provokes the damage of secondary CMCs during reperfusion therapy in AMI cases.9)
The redox system is mainly composed of thioredoxins (TRxs), TRx reductase, thioredoxin interacting protein (TRxNip), and peroxiredoxins (PRxs).10) Previously, we described the temporal expression patterns of the TRx system and their relations to cellular apoptosis in endothelial cells, in the hope that this would provide optimal conditions or time points for TRx system gene or protein delivery in cells and animal models to minimize TRx exhaustion under hypoxia.11) PRx family members are thiol-specific antioxidant proteins, and are also referred to as TRx peroxidases and alkyl-hydroperoxide-reductase-C22 proteins.12) These enzymes are truly ubiquitous and have been identified in yeast, plant and animal cells. PRxs exert their protective antioxidant role in cells through their peroxidase activities (ROOH+2e- → ROH+H2O), which are responsible for the reductions and detoxification of hydrogen peroxide, peroxynitrite, and a wide range of organic hydroperoxides (ROOH).12)13)
Six PRx isoforms have been identified in mammals. However, the temporal expression patterns and functional significances of these isoforms in cell lines found in cardiovascular tissue, especially under conditions of hydrogen peroxide induced oxidative stress, have not yet been elucidated. Therefore, we aimed to determine the temporal expression patterns of the 6 PRxs isoforms in neonatal rat cardiomyocytes (rCMCs), rat vascular smooth muscle cells (rVSMCs), and human umbilical vein endothelial cells (HUVECs) exposed to hydrogen peroxide induced oxidative stress. In addition, we also examined the functional significance of PRx1 overexpression using the lentivirus vector in rCMCs exposed to hydrogen peroxide induced oxidative stress. Changes in molecular pathways associated with cell survival and apoptosis in rCMCs exposed to the same conditions were also examined to further explore the causative relation between apoptosis reduction and PRx1 overexpression.
Isolation and primary cultures of rCMCs were performed using a modified version of a previously reported protocol.14)15) The hearts of 2 to 3 day-old rats (Sprague-Dawley, Orient Bio Inc., Seongnam, Korea) were removed and the left ventricles were collected, washed 3 times with cold ADS buffer (in 116 mM NaCl, 20 mM HEPES, 0.8 mM NaH2PO4, 5.6 mM glucose, 5.4 mM KCl, 0.8 mM MgSO4, pH 7.4), chopped finely with surgical scissors, and digested 3 times for 20 minutes with collagenase/pancreatin (0.56 mg/0.3 mg/mL). The obtained cells were collected selectively and enriched by differential centrifugation through a discontinuous Percoll (Amersham Pharmacia Biotech, Piscataway, NJ, USA) gradient with densities of 1.050, 1.062 and 1.082 g/mL.16) The band at the 1.062/1.082 density interface was collected and used as the rCMCs source. Cells were washed and suspended in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Rockville, MD, USA) supplemented with medium 199 (M199) (Sigma-Aldrich Co., St. Louis, MO, USA), 10% horse serum, 5% fetal bovine serum (FBS), 5000 U/L of streptomycin and 5000 U/L of penicillin. rCMCs were then seeded at 5×105 per well on 2% gelatin-coated (Sigma-Aldrich Co.) 60 mm Primarian plastic culture six-well plate dishes (BD Sciences, San Jose, CA, USA). Media changes were performed initially once daily with serum free media containing DMEM/M199 (4 : 1). rCMCs were cultured at 37℃ in 5% CO2 for 2 to 3 days before being exposed to experimental conditions. With this method, we were able to specifically select rCMCs with a negligible amount of red blood cells or fibroblasts. Purity of rCMCs was more than 98% in morphometric analysis, and immunohistochemical stating with MHC (Santa Cruz Biotechnology, Santa Cruz, CA, USA). All experiments were repeated 3 to 5 times and a total of 90 neonatal rats were used.
Rat vascular smooth muscle cells from the intima-media complex of thoracic aortas of 8-week-old male Sprague-Dawley rats (Orient Bio Inc.) were prepared as previously described.16) We used 5 Sprague-Dawley rats for obtaining rVSMC and repeated this procedure at least 3 to 5 times for robustness. The cells were expanded in 60 mm Primarian plastic culture six-well plates in DMEM supplemented with 10% of FBS at 37℃ in 5% CO2.
We used commercialized HUVECs (ATCC, Chicago, IL, USA) according to the manufacturer's protocol, and cells were obtained and processed as described previously.17) Briefly, umbilical cords were immersed in a culture dish, both umbilical veins were cannulated, and cord ends were bound. Veins were then washed using sterile phosphate buffered saline (PBS) with 0.02% collagenase, and placed in a CO2 incubator for 10 minutes at 37℃. HUVECs were isolated in centrifugation tubes by washing vessels twice with 10 mL M199. The cell suspensions were centrifuged at 1200 rpm for 10 minutes and the supernatants were discarded. The cells were then resuspended using the EGM-2 BulletKit system (Lonza Ltd., Basel, Switzerland) in 2% gelatin-coated six-well plates. Cells were passaged until confluent, dissociated with 0.05% trypsin, and grown for 3 days in fresh medium containing M199 supplemented with 10% FBS and antibiotics using the EGM-2 BulletKit system at 37℃ under 5% CO2.
Cultured rCMCs, rVSMCs, and HUVECs were processed using the Annexin V: Phycoerythrin (PE) Apoptosis Detection kit I (BD Sciences) to assess cell membrane integrity. 7-Aminoacetinomycin D (7-AAD) (BD Sciences) was used as a marker for dying or dead cells. This technique used was based on assessments of cell membrane permeability and on the intercalation of double-stranded DNA. In brief, collected rCMCs, rVSMCs, and HUVECs were washed twice with cold PBS and then resuspended in 1X binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4) at a concentration 5×106 cell/mL. These mixtures were then transferred to a 5 mL culture tube and 5 µL of Annexin-V and 5 µL of 7-AAD were added. After gentle vortexing, the 3 cell lines were incubated for 10 minutes at room temperature in the dark and analyzed using a flow cytometry analysis (FACS Calibur-S, BD Sciences) equipped with an argon ion laser system using excitation and emission wavelengths of 488 and 578 nm, respectively.
To examine the expression patterns of PRx isoforms in rCMCs, rVSMCs, and HUVECs stressed by hydrogen peroxide, western blotting was performed at baseline, and at 30 and 120 minutes after adding 0.25 mM hydrogen peroxide. Harvested rCMCs, rVSMCs, and HUVECs were immersed in PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Seongnam, Korea) and incubated at 4℃ for 2 hours. Extracts were then centrifuged at 4℃ for 15 minutes at 12000 rpm to remove insoluble material. Bradford assays were then used to quantify protein expression.18) Protein concentrations were determined using a dye-binding assay (Bio-Rad protein assay kit, Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of total protein (20 µg) were diluted with sample buffer containing 100 mM dithiothreitol and heated at 98℃ for 5 minutes, then subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a gel apparatus (Hoefer Scientific, San Francisco, CA, USA). Samples were subsequently transferred to polyvinylidene fluoride (PVDF) membranes, and the blots were blocked with 5% skim milk in tris-buffered saline Tween-20 (TBST) for 2 hours. Membranes were then incubated with antibodies against PRx isoforms 1-6 (AbFRONTIER, Seoul, Korea), Bcl-2, Bax, caspase 3, and survivin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1 : 2000 in TBST under shaking at room temperature for 1 hour. Membranes were then washed with TBST, incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1 : 2000-1 : 5000) for 1 hour, and treated with West-Zol kits (iNtRON Biotechnology) for 1 minute. Chemiluminescence was detected using a LAS-3000 (Fujifilm, Minato, Tokyo) for 10-300 seconds. Optical densities (OD) were calculated using the MultiGauge Ver3.1 (Fujifilm) program. Experiments were repeated 5 times and the results are expressed as mean±SD OD as percentages of baseline values. Percentage changes in OD with time versus baseline were also calculated.
HIV-based lentivirus stocks were generated by transient plasmid transfection into 293T cells as described previously.19) PRx1 cDNA was amplified using 5'-CGCGAATTCATGTCTTCAGGAAATGCA-3' and 5'-CGCCTCGAGTCACTTCTGCTTAGAGAA-3' using Taq polymerase. The commentary DNA obtained was subcloned with the 5' end into the EcoRI and the 3' end into the XhoI restriction site at pRRL-cPPT-CMV-X-PRE-SIN containing lentivirus vector (LeV), and packaging was performed by transient transfection. The day prior to transfection, confluent 100 mm plates of 293T cells were split. pRRL-based LeVs were generated by calcium phosphate co-transfection of the transfer vector, the HIV Gap/Pol packaging construct, a Rev expression plasmid, and the VSV-G expression plasmid into 293T cells. To each 15 cm diameter dish, 23 µg transfer vector, 15 µg pMDL-g/pRRE packaging plasmid, 11.5 µg pRSV-REV, and 8 µg pCMV VSV-G envelope were added. DNA was resuspended in 450 µL 0.1×TE (1×TE=10 mM Tris pH 8.0, 1 mM EDTA), 50 µg of 2.5 M CaCl2 were added, and mixtures were incubated at room temperature for 10 minutes. The DNA/CaCl2 solution was then added dropwise to 500 µL (2X) HEPES-buffered saline under vigorous bubbling.
Once it was slightly turbid, the solution was immediately added to the cells. All transfection procedures were conducted over 16 hours, and this was followed by media replacement and virus collection for 48-72 hours. Viral supernatant from 80 plates was filtered through 0.22 µm pore filters and stored at 4℃. The virus was concentrated by ultracentrifugation for 2 hours at 25000 rpm. Virus pellets were resuspended in 1 mL phosphate-buffered saline (PBS) and stored at -80℃.
Rat cardiomyocytes were transfected with lentiviral vector system containing PRx1 gene (LeV-PRx1) or lentiviral vector system contain-ing the green fluorescence protein gene (LeV-GFP) at 1.5×107 IU in the presence of 10 µg/mL DEAE (diethylaminoethyl cellulose) dextran. Media were changed 16 hours after infection and cells were cultured for another 48 hours. To generate oxidative stress, hydrogen peroxide (0.1 or 0.25 mM) was added to cells for 2 hours. rCMCs (3×105 cells) responses were compared under 3 conditions, that is, LeV-PRx1 or LeV-GFP after exposure to hydrogen peroxide and LeV-GFP not exposed to hydrogen peroxide injury.
To examine the transfection efficiency of LeV-PRx1 in vitro, cultured rCMCs were transfected with LeV-PRx1 and stained for PRx1. In brief, cells were cultured in 6 well chamber slides, transfected with LeV-PRx1, and compared with untransfected control cells. The media was changed 16 hours after transfection and cells were cultured for another 48 hours. Cells were then treated with 0.1 or 0.25 mM hydrogen peroxide for 2 hours, washed with PBS to remove hydrogen peroxide and the media was changed. After 48 hours, these rCMCs were fixed with 4% paraformaldehyde, washed with PBS twice for 5 minutes each and blocked with 1% horse serum for 1 hour. Cells were washed again three times with PBS, and incubated with primary antibody for anti-PRx1 (1 : 1000 in PBS, AbFRONTIER) overnight at 4℃. Cells were then rewashed twice with PBS for 5 minutes, incubated with secondary anti-rabbit Texas Red 596 nm (1 : 200 in PBS) for 1 hour, rewashed, and stained with TO-PRO-3 iodide (a nuclear stain; Invitrogen, Carlsbad, CA, USA). Cells were observed and images were acquired using Confocal Laser Scanning Microscope (Olympus BX51, Shinjuku, Tokyo, Japan).
Results are expressed as means±SDs. Statistical analysis was performed using the two-tailed Student's t-test or paired t-test using Statistical Package for the Social Sciences version 16.0 (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant when p were <0.05.
Prior to the main experiment, we checked apoptotic fractions in rCMCs, rVSMCs, and HUVECs after treatment with 0.1 mM hydrogen peroxide for 2 hours. Apoptotic fractions were found to be significantly increased in rCMCs and HUVECs following treatment. Apoptotic fractions were expressed as percentages of fractions at baseline, which were set at 100%. The apoptotic fraction of rCMCs increased from 100±7.07% at baseline to 132±16.49% after hydrogen peroxide treatment (p<0.05). rVSMCs were highly resistant to hydrogen peroxide injury, and apoptotic fractions were not significantly changed compared to the baseline (100±10.49% to 105±14.6%). On the other hand, HUVECs were highly susceptible to hydrogen peroxide, and the apoptotic fraction increased to 360.72±35.35% (p<0.05) (Fig. 1).
The expression patterns of the 6 PRx isoforms in rCMCs, rVSMCs, and HUVECs were examined 30 and 120 minutes after adding 0.1 mM hydrogen peroxide to cell media. PRx2 and 3 expressions in rCMCs were downregulated at 120 minutes (PRx2: 32±5.71%, PRx3: 42±7.23%), but PRx 1 and 6 expressions in rCMCs were significantly upregulated at 120 minutes versus baseline (PRx1: 325.7± 54.82% vs. 100±4.12%, PRx6: 225.82±27.34% vs. 100±2.8%, p<0.05). PRx5 expression was upregulated at 30 minutes (285.87±45.12%), but slightly downregulated at 120 minutes (190.42±39.8%). In rVSMCs, the expressions of all PRx isoforms were significantly upregulated (PRx1: 354.12±75.72%, PRx2: 572.3±87.45%, PRx3: 325.6±34.36%, PRx4: 892.89±97.24%, PRx5: 964.48±112.61%, PRx6: 468.78±79.68%) at both time points. In HUVECs, PRx1, 5 were upregulated (PRx1: 125.25±22.65%, PRx5: 135.87±34.51%) but PRx 2, 3, 4, and 6 were not changed significantly until 120 minutes after the addition of hydrogen peroxide (Fig. 2).
Rat cardiomyocytes (3×105) were transfected with LeV-GFP for 16 hours at a multiplicity of infection of 1.5×107 IU. To determine the transfection efficiency achieved using LeV-GFP, we confirmed GFP expression in rCMCs after transfection by fluorescence microscopy. More than 80% of cells were shown to express GFP (Fig. 3).
Apoptotic fractions were significantly increased by treating cells with hydrogen peroxide for 2 hours (148.32±13.34% vs. 100±13.34%, p<0.05). rCMCs/LeV-GFP (rCMCs transfected with LeV-GFP) exposed to hydrogen peroxide had a higher apoptotic fraction than unexposed cells (187.5±25.49% vs. 111.72±11.81%, p<0.05). LeV-PRx1 treatment effectively reduced the apoptotic fraction as compared to LeV-GFP treatment in rCMCs exposed to hydrogen peroxide (72.55±10.19% vs. 187.5±25.49%, p<0.05) (Fig. 4). These results suggest that PRx1 overexpression protected rCMCs from hydrogen peroxide-induced apoptosis.
Peroxiredoxin 1 expression was significantly higher in rCMCs/LeV-PRx1 (rCMCs transfected with LeV-PRx1) not treated with hydrogen peroxide (193.02±28.44%) than in the control, and significantly higher in rCMCs/LeV-PRx1 treated with hydrogen peroxide than in rCMCs/LeV-GFP treated with hydrogen peroxide (221.02±27.38 vs. 161.01±19.3%, p<0.05) (Fig. 5). Furthermore, rCMCs/LeV-PRx1 expressed significantly more PRx1 than rCMCs/LeV-GFP after treatment with hydrogen peroxide (Fig. 6).
The expression of caspase 3 was lower in rCMCs/LeV-PRx1 treated with hydrogen peroxide than in rCMCs/LeV-GFP treated with hydrogen peroxide (94.3±13.72% vs. 132.3±22.36%, p<0.05), but the expression of survivin was higher in rCMCs/LeV-PRx1 treated with hydrogen peroxide than in rCMCs/LeV-GFP treated with hydrogen peroxide (116.15±21.58% vs. 78.08±15.81%, p<0.05) (Fig. 7). Bax/Bcl-2 ratio (a marker of apoptosis/survival) concurred with the above results, and was lower in rCMCs/LeV-PRx1 treated with hydrogen peroxide than in rCMCs/LeV-GFP treated with hydrogen peroxide (68.1±6.67% vs. 172.6±60%, p<0.05) (Fig. 8). These results show that PRx1 overexpression has a protective effect against hydrogen peroxide related apoptosis in rCMCs.
Peroxiredoxin is a member of the peroxidase family, and has been shown to be related to cell proliferation, differentiation, and apoptosis in mammalian cells.24) Mammalian PRx consists of 6 different isoforms that are classified as typical 2-Cys PRx, the other five 2-Cys PRx isoforms have the TRx-dependent peroxidase activity utilizing TRx, thioredoxin reductase, and NADPH as a reducing system. Mammalian PRxs are 20-30 kd in size and vary in terms of their subcellular localizations. PRx 1, 2, 4, and 6 are found in the cytosol, PRx3 in mitochondria, whereas PRx5 has a complicated distribution, and is found in peroxisomes, mitochondria, and the cytosol.25) In particular, PRxs are involved in the enzymatic degradation of hydrogen peroxide, a wide range of organic hydroperoxides and peroxynitrite, using reducing equivalents provided by thiol-containing proteins like TRxs and PRxs.26)27) Growing evidence indicates that ROS play a critical role in many disorders of the cardiovascular system, such as, ischemia-reperfusion injury, myocardial stunning, apoptosis, and arteriosclerosis.20)21) Furthermore, the accumulation of ROS in mitochondria can lead to apoptotic cell death and ROS may also have direct effects on cellular structure and function, including myocardial remodeling and failure.22)23) Under physiological conditions, the toxic effects of ROS can be prevented by scavenging enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GHPx), and catalase, and by non-enzymatic antioxidants. However, when the production of ROS is high, oxidative stress can have harmful effects on the functional and structural integrity of the heart.23)
In our experiments, the expression patterns of the 6 PRx isoforms were found to be distinct in rCMCs, rVSMCs and HUVCEs. Hydrogen peroxide significantly and time dependently increased rCMC and HUVEC apoptosis in 2 hours as seen by the FACS results. HUVECs were found to be most susceptible to hydrogen peroxide induced injury, and apoptosis increased to 360.72±35.35% compared with the baseline. On the other hand, rCMCs showed a 132±16.49% increase in apoptosis after 2 hours of treatment, but, rVSMCs were found to be highly resistant to hydrogen peroxide. In rCMCs, the expression of PRx1 and 6 showed a continual increase at 30 and 120 minutes, whereas the expression of PRx 2 and 3 was downregulated at 120 minutes. The expression of PRx 5 was elevated at 30 minutes but slightly lower than baseline at 120 minutes. In rVSMCs, the expression of all 6 isoforms of PRx was generally elevated after treatment, particularly the expression of PRx 2, 4, and 5. The expression of PRx in HUVECs only slightly changed compared to that observed in rCMCs and rVSMCs. The three cell types appeared to have unique PRx expression patterns in the presence of excessive hydrogen peroxide, although apoptosis and PRx expression patterns generally appeared to be inversely related. rVSMCs were most resistant to hydrogen peroxide in terms of apoptosis, and this resistance may be attributed to elevated and constant PRx isoform expression. On the other hand, HUVECs expressed PRx isoforms at low levels and were highly susceptible to hydrogen peroxide related cellular apoptosis.
Cytosolic PRx1 is a ubiquitously expressed PRx isoform that is the most abundant in mammalian cells. PRx1 has been associated with various cellular functions apparently unrelated to peroxidase activity. For example, it has been independently identified in a Ras-transformed human mammary epithelial cell line after serum stimulation, in a human erythroleukemic cell line, in which it enhanced the activities of natural killer cells, and in stress-stimulated mouse peritoneal macrophages.28) According to our results, PRx1 expression increased with time after hydrogen peroxide treatment, especially in rCMCs. Therefore, we selected PRx1 as a gene delivery candidate to rescue rCMCs from oxidative stress induced apoptosis, which was in line with previous suggestions concerning the importance of PRx1 in oxidatively stressed cells.26-28)
Peroxiredoxin 1 expression in rCMCs was significantly increased by rCMCs/LeV-PRx1 in the absence of hydrogen peroxide and PRx1 was more upregulated in rCMCs/LeV-PRx1 than in rCMCs/LeV-GFP treated with hydrogen peroxide. PRx1 overexpression protected rCMCs against oxidative stress-induced apoptosis and cell death by hydrogen peroxide.13) Furthermore, PRx1 transfected rCMCs treated with hydrogen peroxide were found to have a significantly lower apoptotic cell fraction than rCMCs/LeV-GFP, and this was found to be closely related to the upregulation of the anti-apoptotic proteins Bcl-2 and survivin, as well as to the downregulation of the pro-apoptotic proteins Bax and caspase 3. In addition, consistent with our findings in a previous study, the overexpression of PRx1 was found to protect thyroid cells from hydrogen peroxide-induced apoptosis, which was also found to be associated with Bax downregulation.13)
Antioxidative systems are highly complex networks, which make the direct and real time measurements of levels of antioxidative molecules under oxidative stress almost impossible, because they have short biologic half-lives, are highly volatile, and are vulnerable to other cellular proteins. In the present study, our PRx1 overexpression model effectively reduced hydrogen peroxide related rCMCs apoptosis. To determine the mechanism involved, further study is required to elucidate the interactions between PRx, TRx, TRX reductase, and TRxNip under oxidative stress. Furthermore, a PRx1 knock-down study using a suitable chemical inhibitor or siRNA is required to validate and support our results. These constitute limitations of the present study, as our results do not enable us to comment on the effects of ischemia-reperfusion or oxidative stress in vivo. Also, we were not able to assess the existence of cellular necrosis in hydrogen peroxide-induced oxidative stress. However, apoptosis and its reverse are more important than necrosis in damaged myocardial tissue. Therefore, our apoptosis focus may be justified. More detailed analyses focused on changes in necrosis patterns with PRx gene delivery are also required.
Up to now, there is no clinical study on PRx delivery or overexpression. However, there are a few clinical studies ongoing on TRx inhibitor, especially in the oncology field. Compared to TRx research, PRx studies are not abundant and very limited. Because of the similar action and molecular relation of these two proteins, we surmise that Prx can also be an alternative candidate of human application for myocardial ischemia or oncologic disease. Protein delivery of PRx with regards to thromolysis or emergent coronary intervention for AMI to relieve oxidative damage related to ischemia-reperfusion injury will be helpful.
In summary, PRx1 expression was found to be significantly increased by hydrogen peroxide in rCMCs, and PRx1 overexpression was found to protect rCMCs against oxidative stress-induced apoptosis and cell death by hydrogen peroxide. We conclude that PRx provides an effective target for new drug development aimed at reducing rCMC apoptosis in ischemia-reperfusion situations.
Figures and Tables
Fig. 1
FACS and the detection of rCMC, rVSMC, and HUVEC apoptosis under hydrogen peroxide-induced oxidative stress. Flow cytometric analysis showed significant increases in apoptosis after treatment with 0.1 mM hydrogen peroxide for 2 hours versus non-treated control rCMCs, rVSMCs, or HUVECs. *Compared to control, p<0.05. FACS: flow cytometry analysis, rCMCs: neonatal rat cardiomyocytes, rVSMCs: rat vascular smooth muscle cells, HUVECs: human umbilical vein endothelial cells, Con: control, H2O2: hydrogen peroxide.

Fig. 2
Temporal expression patterns of the 6 PRx isoforms in rCMCs, rVSMCs, and HUVECs exposed to hydrogen peroxide-induced oxidative stress. PRx1 and 5 were overexpressed at 30 and 120 minutes, whereas PRx6 was overexpressed at 120 minutes in rCMCs after hydrogen peroxide stimulation. All isoforms were overexpressed in rVSMCs at both time points. No significant change in the expression of PRx isoforms was observed in HUVECs. *Compared to control, p<0.05. PRx: peroxiredoxin, rCMCs: neonatal rat cardiomyocytes, rVSMCs: rat vascular smooth muscle cells, HUVECs: human umbilical vein endothelial cells, con: control, min: minutes.
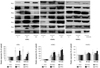
Fig. 3
Confirmation of gene expression after the transfection of LeV-GFP into rCMCs. GFP expression in rCMCs/LeV-GFP. rCMCs (3×105 cell) were transfected with LeV-GFP for 16 hours at a multiplicity of infection of 1.5×107 IU. A: rCMCs appeared healthy under optical microscopy (×200). B: GFP expression in rCMCs transfected with LeV-GFP was observed in over 80% of cells by fluorescence microscopy (×200). LeV-GFP: GFP gene containing lentivirus vector, rCMCs: neonatal rat cardiomyocytes, GFP: green fluorescence protein.
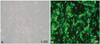
Fig. 4
FACS and the quantitation of apoptosis in rCMCs transfected with LeV-PRx1 or LeV-GFP and exposed to hydrogen peroxide. The transfection of LeV-PRx1 significantly reduced apoptosis induced by 0.25 mM hydrogen peroxide for 2 hours as compared to LeV-GFP transfection (72.55±10.19% vs. 187.5±25.49%, p<0.05). *Compared to control, p<0.05, †Compared to LeV-GFP+H2O2, p<0.05. FACS: flow cytometric analysis, rCMCs: neonatal rat cardiomyocytes, LeV-PRx1: PRx1 gene containing lentivirus vector, LeV-GFP: green fluorescence protein gene containing lentivirus vector, Con: control, H2O2: hydrogen peroxide, rVSMCs: rat vascular smooth muscle cells.

Fig. 5
Expression patterns of PRx1 in rCMCs transfected with LeV-PRx1 in hydrogen peroxide induced oxidative stress. PRx1 expression patterns under various conditions of hydrogen peroxide induced oxidative stress and PRx1 gene delivery. Cells were exposed to 0.25 mM hydrogen peroxide for 2 hours. LeV-PRx1 transfection significantly upregulated PRx1 expression versus LeV-GFP transfection in hydrogen peroxide treated rCMCs. *Compared to control, p<0.05, †Compared to LeV-GFP+H2O2, p<0.05. rCMCs: rat cardiomyocytes, LeV-PRx1: PRx1 gene containing lentivirus vector, con: control, H2O2: hydrogen peroxide, LeV-GFP: green fluorescence protein gene containing lentivirus vector.
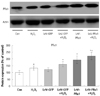
Fig. 6
Immunohistochemistry in rCMCs transfected with LeV-PRx1. PRx1 expression was upregulated in rCMCs/LeV-PRx1 as compared to rCMCs/LeV-GFP treated with 0.25 mM hydrogen peroxide for 2 hours. Cytolsolic isoforms, PRx1 was found to be highly expressed in the rCMCs/LeV-PRx1 (stained red). rCMCs: neonatal rat cardiomyocytes, LeV-PRx1: PRx1 gene containing lentivirus vector, LeV-GFP: green fluorescence protein gene containing lentivirus vector.

Fig. 7
Protein expression of caspase 3 and survivin in rCMCs transfected with LeV-Prx1. LeV-PRx transfection effectively reduced caspase 3 expression and enhanced survivin expression in rCMCs treated with 0.25 mM hydrogen peroxide for 2 hours. *Compared with control, p<0.05, †Compared with LeV-GFP+H2O2, p<0.05. rCMCs: rat cardiomyocytes, LeV-PRx1: PRx1 gene containing lentivirus vector, Con: control, H2O2: hydrogen peroxide, LeV-GFP: GFP gene containing lentivirus vector.

Fig. 8
Bax/Bcl-2 ratios in LeV-PRx1 versus LeV-GFP transfected rCMCs treated with hydrogen peroxide. Bax/Bcl-2 ratio was significantly lower in LeV-PRx1 than in LeV-GFP transfected rCMCs treated with 0.25 mM hydrogen peroxide for 2 hours. *Compared to control, p<0.05, †Compared to LeV-GFP+H2O2, p<0.05. LeV-PRx1: PRx1 gene containing lentivirus vector, LeV-GFP: GFP gene containing lentivirus vector, rCMCs: rat cardiomyocytes, Con: control, H2O2: hydrogen peroxide, neonatal rat cardiomyocytes.

Acknowledgments
This study was supported by a research grant from the Korean Society of Cardiology in 2008. We cordially appreciate Pf. Seung-Taik Kim in Chungbuk National University Hospital for providing us with the lentiviral vector construct.
References
1. Tuteja N, Singh MB, Misra MK, Bhalla PL, Tuteja R. Molecular mechanisms of DNA damage and repair: progress in plants. Crit Rev Biochem Mol Biol. 2001. 36:337–397.
2. Tuteja N, Tuteja R. Unravelling DNA repair in human: molecular mechanisms and consequences of repair defect. Crit Rev Biochem Mol Biol. 2001. 36:261–290.
3. Tuteja N, Ahmad P, Panda BB, Tuteja R. Genotoxic stress in plants: shedding light on DNA damage, repair and DNA repair helicases. Mutat Res. 2009. 681:134–149.
4. Kevin LG, Novalija E, Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg. 2005. 101:1275–1287.
5. Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007. 100:460–473.
6. Boersma E, Mercado N, Poldermans D, Gardien M, Vos J, Simoons ML. Acute myocardial infarction. Lancet. 2003. 361:847–858.
7. Byrne JA, Grieve DJ, Cave AC, Shah AM. Oxidative stress and heart failure. Arch Mal Coeur Vaiss. 2003. 96:214–221.
8. Ferrari R, Guardigli G, Mele D, Percoco GF, Ceconi C, Curello S. Oxidative stress during myocardial ischaemia and heart failure. Curr Pharm Des. 2004. 10:1699–1711.
9. Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987. 84:1404–1407.
10. Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009. 15:RA209–RA219.
11. Park KJ, Kim YJ, Choi EJ, et al. Expression pattern of the thioredoxin system in human endothelial progenitor cells and endothelial cells under hypoxic injury. Korean Circ J. 2010. 40:651–658.
12. Chae HZ, Robison K, Poole LB, Church G, Storz G, Rhee SG. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci U S A. 1994. 91:7017–7021.
13. Hofmann B, Hecht HJ, Flohé L. Peroxiredoxins. Biol Chem. 2002. 383:347–364.
14. Poindexter BJ, Smith JR, Buja LM, Bick RJ. Calcium signaling mechanisms in dedifferentiated cardiac myocytes: comparison with neonatal and adult cardiomyocytes. Cell Calcium. 2001. 30:373–382.
15. Kobayashi S, Lackey T, Huang Y, et al. Transcription factor gata4 regulates cardiac BCL2 gene expression in vitro and in vivo. FASEB J. 2006. 20:800–802.
16. Kau TR, Schroeder F, Ramaswamy S, et al. A chemical genetic screen identifies inhibitors of regulated nuclear export of a forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003. 4:463–476.
17. Hoshi H, McKeehan WL. Brain- and liver cell-derived factors are required for growth of human endothelial cells in serum-free culture. Proc Natl Acad Sci U S A. 1984. 81:6413–6417.
18. Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997. 95:320–323.
19. Zeng L, Planelles V, Sui Z, et al. HIV-1-based defective lentiviral vectors efficiently transduce human monocytes-derived macrophages and suppress replication of wild-type HIV-1. J Gene Med. 2006. 8:18–28.
20. Chen HW, Chien CT, Yu SL, Lee YT, Chen WJ. Cyclosporine A regulate oxidative stress-induced apoptosis in cardiomyocytes: mechanisms via ROS generation, iNOS and Hsp70. Br J Pharmacol. 2002. 137:771–781.
21. Penna C, Mancardi D, Tullio F, Pagliaro P. Postconditioning and intermittent bradykinin induced cardioprotection require cyclooxygenase activation and prostacyclin release during reperfusion. Basic Res Cardiol. 2008. 103:368–377.
22. Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000. 192:1001–1014.
23. Ellis HR, Poole LB. Novel application of 7-chloro-4-nitrobenzo-2-oxa-1, 3-diazole to identify cysteine sulfenic acid in the AhpC component of alkyl hydroperoxide reductase. Biochemistry. 1997. 36:15013–15018.
24. Hirotsu S, Abe Y, Okada K, et al. Crystal structure of a multifunctional 2-Cys peroxiredoxin heme-binding protein 23 kDa/proliferation-associated gene product. Proc Natl Acad Sci U S A. 1999. 96:12333–12338.
25. Hofmann B, Hecht HJ, Flohé L. Peroxiredoxins. Biol Chem. 2002. 383:347–364.
26. Wood ZA, Schröder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003. 28:32–40.
27. Prospéri MT, Ferbus D, Karczinski I, Goubin G. A human cDNA corresponding to a gene overexpressed during cell proliferation encodes a product sharing homology with amoebic and bacterial proteins. J Biol Chem. 1993. 268:11050–11056.
28. Izumi Y, Kim-Mitsuyama S, Yoshiyama M, et al. Important role of apoptosis signal-regulating kinase 1 in ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2005. 25:1877–1883.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download