Abstract
Background and Objectives
Lesions of vascular bifurcation and their treatment outcomes have been evaluated by anatomical and physiological methods, such as intravascular ultrasound and fractional flow reserve (FFR). However, local changes in flow dynamics in lesions of bifurcation have not been well evaluated. This study aimed at evaluating changes in the local flow patterns of bifurcation lesions.
Materials and Methods
Eight (n=8) representative simulation-models were constructed: 1 normal bifurcation, 5 main-branch (MB) stenting models with various side-branch (SB) stenoses (ostial or non-ostial 75% diameter stenosis with 1- or 2-cm lesion lengths, ostial 75% diameter stenosis caused by carina shift), and 2 post-kissing models (no or 50% SB residual stenosis). Pressure, velocity, and wall shear stress (WSS) profiles around the bifurcation sites were investigated using computational fluid dynamics.
Results
Post-stenting models revealed significant pressure drop in the SB (FFR<0.75), excluding the carina shift model (FFR=0.89). In the post-kissing models, there was no significant pressure drop. All post-stenting models revealed eccentric low velocity flow patterns and areas of low WSS, primarily in the lateral wall on distal MB. Post-kissing angioplasty improved pressure drop in the SB but resulted in alteration of flow distribution in the MB. In the carina shift model, kissing ballooning resulted in deteriorated local flow conditions due to increased area of low velocity and WSS.
Percutaneous coronary interventions using stents are the most widely used techniques for the treatment of coronary arterial disease, and 15-20% of treated coronary lesions involve bifurcation.1)2) Clinical trials designed to evaluate treatment options for coronary bifurcated lesions with multiple stents have failed to prove the benefit of systematic 2-stenting techniques,3)4) and study results for dedicated bifurcation stents are still forthcoming, although they appear promising.5)6) As such, side-branch (SB) intervention remains the most common strategy to treat coronary bifurcated lesions.
Local hemodynamics, including low or oscillatory shear stress, are known to be associated with cellular proliferation, inflammation and thrombosis.7) Coronary atheromatous plaques and neointimal hyperplasia following coronary intervention tend to form in coronary artery bifurcation, where normal blood flow patterns are disturbed.8)9) Altered coronary vascular geometry and associated blood flow hemodynamic disturbances occurring after stent implantation have been reported as possible restenosis mechanisms.10)11) However, there exist a few reports on the impact of commonly used provisional SB intervention techniques on local blood flow hemodynamics. Computational fluid dynamics (CFD) is an advanced simulation technique that analyzes hemodynamic data, and is well suited for this purpose.
The aim of in this study was to conduct CFD analysis to evaluate local hemodynamic alterations following main branch (MB) stenting and/or subsequent SB angioplasty by using representative computational models.
Computational domains for 8 models of bifurcated lesions were constructed using a commercial numerical package (FLUENT 6.2, Fluent Inc., NH, USA) for the investigation of hemodynamics (pressure, velocity, and WSS profiles) around coronary bifurcation. MB and SB configurations were derived by Finet's law with a fractal ratio of 0.678.12) The angle of MB and SB was assumed to be 45°, to represent the most prevalent site of bifurcated lesion between the left anterior descending coronary artery and the first diagonal branch.13)14) The geometry of stenosis generated was based on an ellipse, with aspect ratio defined as the length of the lesion to the diameter of the lesion. By applying symmetrical condition to the yz-plane shown in Fig. 1, we solved the governing equations (continuity and momentum) of steady-state fluid flow. To consider myocardial physiology, micro-vessels were assumed to be porous media that mimicked pressure drop from arterial pressure to venous pressure (10 mmHg).15) As a boundary condition, the inlet pressure of the MB was defined as 110 mmHg, and a venous pressure of 10 mmHg was applied to the outlet of the micro-vessels. The rheological property of blood was assumed to be Newtonian fluid, with a density of 1,060 kg/m3 and viscosity of 0.004 Pa·s (Fig. 1).
The models used in this study are illustrated in Fig. 2. Model A is a normal bifurcation model, and models B to F are post-MB stenting models. Fig. 2B and C show post-MB stenting models with native ostial SB lesions due to circular plaques with 75% diameter stenosis and lesion lengths of 1 and 2 cm. Fig. 2D and F illustrate the post-MB stenting models with non-ostial SB lesions of 1 and 2 cm lesion lengths. Model F represents ostial stenosis mainly due to carina shift with 75% diameter stenosis and 50% area stenosis.16) Fig. 2G and F illustrate the post-kissing models. In Fig. 2G, the SB ostium does not have residual stenosis, and in Fig. 2H, the SB ostium has a 50% residual stenosis.
The pressure profiles were calculated by CFD simulation for assessment of pressure drop after the bifurcation. The fractional flow reserve (FFR) is a quantitative physiologic parameter representing the fraction of maximal myocardial flow.17)18) In practice, FFR can be obtained by calculating the ratio of distal coronary pressure to proximal aortic pressure.17-20) In this study, FFR was defined as the ratio of the pressure 1 cm distal to the SB from the site of bifurcation to the pressure 1 cm proximal to the MB. Coronary flow velocity and WSS were calculated by CFD simulations as qualitative coronary flow parameters.
Static pressure profiles are shown in Fig. 3. In the normal bifurcation model, there was no pressure drop in the bifurcation (Fig. 3A). Models with 75% diameter stenosis due to circular plaques exhibited significant pressure drop across the stenoses (Fig. 3B-E). Larger pressure drops were observed in non-ostial SB stenotic lesions than in ostial SB lesions, even in those with the same extent of luminal stenosis. Moreover, the impact of lesion length on pressure drop was much less in ostial lesions than in non-ostial lesions. In contrast to circular stenotic models, the carina shift model revealed no significant pressure drop, despite 75% diameter stenosis.
Flow velocity profiles and WSS distributions of each bifurcation model are shown in Figs. 4 and 5. Fig. 4A shows a typical pattern of velocity distribution in a bifurcating vasculature. Velocity profiles in the normal bifurcation model are skewed toward the carina, resulting in higher velocity along the medial side walls (yellow and red) and lower velocity along the lateral side walls (green and blue). Post-stenting models with SB ostial stenosis (model B and C) introduced a more heterogeneous velocity distribution in MB and SB. In the SB, high velocity jet was created in the lateral walls, and low velocity flow was created along the medial sidewalls. In the distal MB, low velocity flow became more profound due to over-expansion caused by stent implantation. In the post-kissing ballooning angioplasty model without residual stenosis (model G), the SB velocity profile was similar to that of the normal bifurcation model. However, low velocity flow was demonstrated in both proximal and distal over-expanded segments.
Images of WSS profiles in the 8-CFD models are shown in Fig. 5, and areas of low WSS, defined as WSS less than 4 Pa, are described in Table 1. The total area of low WSS was minimal in the normal bifurcation model (green and blue areas in Fig. 5A, 8.02 mm2, 1.8%). In post-stenting bifurcation models with ostial SB stenosis (Fig. 5B and C), the total area of low WSS was increased, particularly at the MB lateral wall and SB medial wall, with values of 119.4 mm2 (26.6%) and 108.6 mm2 (24.8%), respectively. Post-stenting models with non-ostial SB stenosis exhibited low WSS areas in the SB ostial region before SB stenosis, and at the MB lateral wall. In models with SB stenosis (Fig. 5B-E), low WSS areas were pronounced in the SB. In the carina shift model (Fig. 5F), the total area of low WSS was smaller than that of other SB stenosis models (39.3 mm2, 8.6%). Total area of low WSS in the post-kissing models was smaller than that of the SB stenosis models, but larger than that of the normal bifurcation model: 56.8 mm2 (12.4%) in model G and 92.7 mm2 (20.5%) in model H.
Coronary bifurcation lesion remains one of the most challenging lesions to manage in the field of percutaneous coronary intervention.5)21-24) The reported restenosis and target vessel revascularization rates are still high.25)26) Provisional SB angioplasty and/or restenting after MB stenting are the most common strategies to treat bifurcation lesions,27) but this technique may alter vascular geometry and local hemodynamic profiles. Low or abnormal WSS is reported to be associated with atherosclerosis progression.8)
In this study, we evaluated the hemodynamic profiles in terms of pressure, velocity, and WSS in various representative bifurcation models by using CFD simulation. The representative bifurcation lesion models used in this study included a normal bifurcation, 5 post-MB stenting models, and 2 post-kissing balloon angioplasty models. We thought that these models represent situations commonly encountered during percutaneous interventions of coronary bifurcation lesions.
In post-MB stenting models, the carina shift model (Model F) revealed relatively small pressure drop, although this lesion exhibited similar angiographic stenosis as other post-MB stenting models with SB stenosis (Models B to E). This finding can be explained by the fact that the carina shift model had a larger lumen area than native atherosclerotic plaque models, as previously reported by Koo et al.16) Interestingly, this study revealed different pressure drop patterns by lesion location. When the lesion length was doubled, the pressure drop was more prominent in non-ostial SB lesions than in ostial lesions (Fig. 3D and E). This finding implies that a lesion's functional significance is influenced by its morphology, which cannot be fully assessed by quantitative coronary angiographic parameters. Thus, SB lesion location should be regarded as a determinant of functional significance.
The distribution of flow velocity and WSS was similar to that reported previously.28) However, this study included more types of bifurcation lesion models that simulated diverse clinical situations. In our study, 3 different types of SB lesions (native ostial or non-ostial SB lesions and ostial SB lesion caused by carina shift) were evaluated. In all models, low WSS areas were mainly located in an over-expanded segment of distal MB. Thus, when a natural bifurcation vascular geometry was altered by MB stenting, additional intervention for SB stenosis did not improve local hemodynamic derangement. Therefore, avoidance of over-expansion and performance of proximal optimization may be required to minimize local flow disturbances.
This study has limitations. First, the inlet condition applied in this study was static pressure flow. However, the result of CFD simulation in this study was similar to that reported previously using pulsatile flow models.28) Second, the models used in this study were simplified and not patient-specific. This problem can be solved in the near future by incorporating computed tomographic findings into CFD simulations.
In our study, the area of low WSS was minimal in the carina shift model. This finding suggests that additional intervention for functionally insignificant jailed SB lesions caused by carina shift may worsen local hemodynamic conditions and clinical outcomes. This result may partially explain why kissing ballooning to SB did not improve, or alternatively, may have even worsened the outcomes of recent clinical trials.29)30)
Figures and Tables
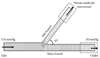 | Fig. 1Bifurcation lesion modeling with computational fluid dynamics. A normal bifurcation model was created using Finet's law and a typical bifurcation angle. As the boundary condition, inlet pressure of the main branche was defined as 110 mmHg, and venous pressure of 10 mmHg was applied to the outlet of the micro-vessels. To consider the physiology of the myocardium, micro-vessels were assumed as porous media that mimicked the pressure drop from arterial pressure to venous pressure (10 mmHg). The rheological property of blood was assumed as Newtonian fluid with a density of 1,060 kg/m3 and viscosity of 0.004 Pa·s. |
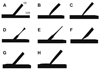 | Fig. 2Representative computational fluid dynamics models. A: normal bifurcation. B and C: post-MB stenting models with various SB stenoses. F: post-MB stenting model with carina shift. G and H: post-kissing balloon angioplasty models with or without SB residual stenosis. MB: main branch, SB: side branch. |
 | Fig. 3Static pressure profiles in the 8-computational bifurcation models. There was no significant pressure drop in the normal bifurcation model (A). The post-MB stenting models are shown in (B) to (E). Post-MB stenting models with native ostial (B and C) and, non-ostial (D and E) SB stenosis revealed significant pressure drops in the SB. Regarding the carina shift model (F), the pressure drop was lower than those in the native SB stenosis models (B-E). After the kissing ballooning in the SB lesion, the pressure of SB was restored to the level of the distal MB pressure (G). Regarding residual stenosis after kissing ballooning (H), the pressure of SB was higher than that in the models with post-stenting SB lesions (B-E). *FFR is defined as the ratio of PSB/PMB. FFR: fractional flow reserve, MB: main branch, SB: side branch. |
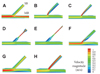 | Fig. 4Velocity profiles in computational bifurcation models. Velocity patterns in the bifurcation site were typical in the normal bifurcation model (A). Velocity profiles were skewed toward the carina, resulting in high velocity along the medial walls (yellow and red) and low velocity along the outer lateral walls (green and blue). In post-MB stenting models with significant SB stenosis, high velocity jet flows were observed at the site of stenosis, and low velocity areas were located in the lateral MB walls and medial SB walls (B-F). In post-kissing models (G and H), the low velocity area in SB was smaller than that in post-MB stenting models, but a larger low velocity area in the MB lateral wall was still observed relative to the area in the normal bifurcation model. MB: main branch, SB: side branch. |
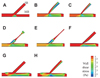 | Fig. 5Wall shear stresses in the 8-computational bifurcation models. In the normal bifurcation model (A), a small low WSS area was observed along the lateral side walls (green and blue). In post-MB stenting models with significant SB stenosis (B-E), low WSS areas were observed in the SB and lateral side MB wall. Low WSS areas in the lateral side MB were also more exaggerated in the post-kissing balloon angioplasty models (G and H) and carina shift model (F). MB: main branch, SB: side branch, WSS: wall shear stress. |
Acknowledgments
This study was supported by grants from the Cardiology (2009-2), the Clinical Research Center for Ischemic Heart Disease, Seoul, the Republic of Korea (0412-CR02-0704-0001) and the Innovative Research Institute for Cell Therapy, Seoul National University Hospital (A062260), sponsored by the Ministry of Health, Welfare & Family, Republic of the Republic of Korea.
References
1. Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation. 2010. 121:e46–e215.
2. Sharma SK, Mares AM, Kini AS. Coronary bifurcation lesions. Minerva Cardioangiol. 2009. 57:667–682.
3. Ferenc M, Gick M, Kienzle RP, et al. Randomized trial on routine vs. provisional T-stenting in the treatment of de novo coronary bifurcation lesions. Eur Heart J. 2008. 29:2859–2867.
4. Colombo A, Bramucci E, Sacca S, et al. Randomized study of the crush technique versus provisional side-branch stenting in true coronary bifurcations: the CACTUS (Coronary Bifurcations: Application of the Crushing Technique Using Sirolimus-Eluting Stents) Study. Circulation. 2009. 119:71–78.
5. Colombo A, Moses JW, Morice MC, et al. Randomized study to evaluate sirolimus-eluting stents implanted at coronary bifurcation lesions. Circulation. 2004. 109:1244–1249.
6. Koo BK, Fitzgerald PJ. Novel coronary stent platforms. Korean Circ J. 2008. 38:393–397.
7. Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007. 49:2379–2393.
8. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999. 282:2035–2042.
9. Stone PH, Coskun AU, Kinlay S, et al. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and instent restenosis in humans: in vivo 6-month follow-up study. Circulation. 2003. 108:438–444.
10. LaDisa JF Jr, Olson LE, Molthen RC, et al. Alterations in wall shear stress predict sites of neointimal hyperplasia after stent implantation in rabbit iliac arteries. Am J Physiol Heart Circ Physiol. 2005. 288:H2465–H2475.
11. Murata T, Hiro T, Fujii T, et al. Impact of the cross-sectional geometry of the post-deployment coronary stent on in-stent neointimal hyperplasia: an intravascular ultrasound study. Circ J. 2002. 66:489–493.
12. Finet G, Gilard M, Perrenot B, et al. Fractal geometry of arterial coronary bifurcations: a quantitative coronary angiography and intravascular ultrasound analysis. EuroIntervention. 2008. 3:490–498.
13. Pflederer T, Ludwig J, Ropers D, Daniel WG, Achenbach S. Measurement of coronary artery bifurcation angles by multidetector computed tomography. Invest Radiol. 2006. 41:793–798.
14. Wang JC, Normand SL, Mauri L, Kuntz RE. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation. 2004. 110:278–284.
15. Yull Park J, Young Park C, Mo Hwang C, Sun K, Goo Min B. Pseudo-organ boundary conditions applied to a computational fluid dynamics model of the human aorta. Comput Biol Med. 2007. 37:1063–1072.
16. Koo BK, Waseda K, Kang HJ, et al. Anatomic and functional evaluation of bifurcation lesions undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2010. 3:113–119.
17. Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996. 334:1703–1708.
18. Pijls NH. Optimum guidance of complex PCI by coronary pressure measurement. Heart. 2004. 90:1085–1093.
19. Suh JW, Koo BK, Jo SH, et al. Optimal dosage and method of administration of adenosine for measuring the coronary flow reserve and the fractional flow reserve in Koreans. Korean Circ J. 2006. 36:300–307.
20. Park KH, Koo BK, Shu JH, et al. Assessment of intermediate coronary stenosis in Koreans using the fractional flow reserve. Korean Circ J. 2008. 38:468–474.
21. Ikeno F, Kim YH, Luna J, et al. Acute and long-term outcomes of the novel side access (SLK-View) stent for bifurcation coronary lesions: a multicenter nonrandomized feasibility study. Catheter Cardiovasc Interv. 2006. 67:198–206.
22. Lefevre T, Ormiston J, Guagliumi G, et al. The Frontier stent registry: safety and feasibility of a novel dedicated stent for the treatment of bifurcation coronary artery lesions. J Am Coll Cardiol. 2005. 46:592–598.
23. Ormiston J, Webster M, El-Jack S, McNab D, Plaumann SS. The AST petal dedicated bifurcation stent: first-in-human experience. Catheter Cardiovasc Interv. 2007. 70:335–340.
24. Sharma SK, Kini AS. Coronary bifurcation lesions. Cardiol Clin. 2006. 24:233–246.
25. Louvard Y, Lefevre T, Morice MC. Percutaneous coronary intervention for bifurcation coronary disease. Heart. 2004. 90:713–722.
26. Melikian N, Di Mario C. Treatment of bifurcation coronary lesions: a review of current techniques and outcome. J Interv Cardiol. 2003. 16:507–513.
27. Steigen TK, Maeng M, Wiseth R, et al. Randomized study on simple versus complex stenting of coronary artery bifurcation lesions: the Nordic bifurcation study. Circulation. 2006. 114:1955–1961.
28. Williams AR, Koo BK, Gundert TJ, Fitzgerald PJ, LaDisa JF Jr. Local hemodynamic changes caused by main branch stent implantation and subsequent virtual side branch balloon angioplasty in a representative coronary bifurcation. J Appl Physiol. 2010. 109:532–540.
29. Niemela M, Kervinen K, Erglis A, et al. Randomized comparison of final kissing balloon dilatation versus no final kissing balloon dilatation in patients with coronary bifurcation lesions treated with main vessel stenting: the Nordic-Baltic Bifurcation Study III. Circulation. 2011. 123:79–86.
30. Gwon HC, Choi SH, Song YB, et al. Long-term clinical results and predictors of adverse outcomes after drug-eluting stent implantation for bifurcation lesions in a real-world practice: the COBIS (Coronary Bifurcation Stenting) registry. Circ J. 2010. 74:2322–2328.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download