Abstract
Stent fracture is likely to be caused due to mechanical stress at the hinge point or kinking movement at the point of aneurysm formation with stent malapposition. To our knowledge, this is the first published report of stent fracture at the proximal shaft of the left main stem in a patient with acute myocardial infarction.
The suggested predisposing factors associated with fractures of coronary stent are sites of increased vessel movement, lengthy coronary stents, right coronary artery stents, overlapping stents, sirolimus-eluting stents (SES), and saphenous vein graft stents.1)2) In addition, stent fracture is likely to be caused due to bending movement such as torsion, flexion, and rotation at the hinge point or kinking movement at the point of aneurysm formation with stent malapposition.3) We report the first case of stent fracture at the proximal shaft of the left main stem (LMS).
A 55-year-old man visited our hospital for follow-up coronary angiography. He had no particular symptoms prior to admission, but he wanted to undergo coronary angiography. Smoking was the only cardiovascular risk factor.
Twenty-one months ago, primary percutaneous coronary intervention was performed because of symptoms of progressive angina and ST-segment elevation on the electrocardiogram. He was diagnosed with acute myocardial infarction (AMI) with the culprit lesion being a long stenotic lesion involving the segment from the ostium of the LMS to the mid left anterior descending coronary artery (LAD) (Fig. 1A). We implanted SES (Cypher®, Cordis, 3.5×23 mm) from the ostium of the LMS to the proximal LAD and then overlapped paclitaxel-eluting stent (Taxus®, Boston, 3.0×32 mm) at the proximal to mid-LAD. Additional ballooning was also performed, using high-pressure balloon (Quantum®, Schneider, 4.0×12 mm) up to 10 atm for 15 seconds (Fig. 1B). Intravascular ultrasound (IVUS; 42 mHz, Clear View, MA, Boston, USA) confirmed well-positioned stents.
Follow-up coronary angiography showed stent fracture at the proximal shaft of the LMS with minimal luminal narrowing (Fig. 2A and B). IVUS revealed the absence of the stent strut corresponding to stent fracture and ectatic change with malapposition of stent around the site of stent fracture (Fig. 2C, D and E). Based on these findings, the lesion was redilated up to 16 atm for 15 seconds with high-pressure balloon (Quantum®, Schneider, 4.0×12 mm). Finally, IVUS confirmed well-apposite struts.
The suggested predisposing factors associated with stent fracture are sites of increased vessel movement, lengthy coronary stents, right coronary artery stents, overlapping stents, SES, and saphenous vein graft stents.1)2) Fractures occur as a result of several mechanisms including repetitive compression due to bending movement at the hinge points, and kinking movement and shear stress at the point of aneurysm formation with stent malapposition.3)
The predisposing factors associated with the occurrence of ectasia or aneurysm formation and stent malapposition are long stent length, AMI, and chronic total occlusion.4) The mechanisms of ectasia or aneurysm formation and stent malapposition may be related to the intima-media injuries because of oversized high-pressure balloon inflation and patient-specific hypersensitivity to drug-eluting stents.5)6)
A few cases of LMS stent fractures have been reported, and the stent fractures were located at the hinge point of bifurcation of LMS into left circumflex artery or LAD.2)7)8) The stent fracture of the LMS is very rare and is probably caused by rich elasticity of the LMS. Histologically, LMS lacks adventitia and has considerable smooth muscle and elastic tissue. The amount of elastic tissue diminishes distally in the coronary tree; therefore, LMS has the most elastic tissue among the coronary vessels.9)
In our case, the stent fracture occurred at an uncommon site, the proximal shaft of the LMS, and SES and AMI appeared to be the predisposing factors for stent fracture and malapposition. The closed cell design of the SES and thicker metal struts offer some benefits including even strut distribution allowing more predictable local drug delivery and low rate of intimal growth, but make the stent vulnerable to fracture and positive remodeling such as ectatic change or aneurysm formation compared with other drug-eluting stents.10)11) Oversized dilatation of the vessel lumen may cause thrombus compression and displacement of primary stenting in AMI.4)
In our case, ectatic change and malapposition of the SES might have developed at first due to the highly calcified, fixed lesion, and then the stent fracture might have occurred due to the kinking movement. We can conclude that if stent fracture develops at an uncommon site with or without any predisposing factors, ectatic changes associated with stent malapposition should be speculated. Physicians who decide to postdilate an undersized SES during the treatment of AMI should be aware of the risk of strut fractures related with aggressive postdilatation and should consider routine IVUS evaluation of the stent-implanted segments.
Figures and Tables
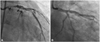 | Fig. 1A: index coronary angiography showed diffuse, irregular narrowing from ostium of the left main stem to the mid left anterior descending coronary artery. B: index coronary angiography showed a good patency without luminal narrowing after percutaneous coronary intervention. |
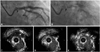 | Fig. 2A: follow-up coronary angiography showed a small filling defect at the proximal shaft of the LMS (indicated by an arrow). B: fluoroscopy showed stent-free lesion at the proximal shaft of the LMS (indicated by an arrow). C: IVUS showed a stent strut at the proximal part of stent fracture and ectatic change with malapposition (indicated by an arrow). D: IVUS showed a stent-free lumen corresponding to stent fracture. E: IVUS showed a stent strut at the distal part of stent fracture and ectatic change with malapposition (indicated by an arrow). LMS: left main stem, IVUS: intravascular ultrasound. |
References
1. Aoki J, Nakazawa G, Tanabe K, et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007. 69:380–386.
2. Lee MS, Jurewitz D, Aragon J, Forrester J, Makkar RR, Kar S. Stent fracture associated with drug-eluting stents: clinical characteristics and implications. Catheter Cardiovasc Interv. 2007. 69:387–394.
3. Doi H, Maehara A, Mintz GS, et al. Classification and potential mechanisms of intravascular ultrasound patterns of stent fracture. Am J Cardiol. 2009. 103:818–823.
4. Hong MK, Mintz GS, Lee CW, et al. Late stent malapposition after drug-eluting stent implantation: an intravascular ultrasound analysis with long-term follow-up. Circulation. 2006. 113:414–419.
5. Aoki J, Kirtane A, Leon MB, Dangas G. Coranary artery aneurysm after drug-eluting stent implantation. JACC Cardiovasc Interv. 2008. 1:14–21.
6. Lee SY, Im E, Yang WI, Kim JS, Cho YH, Shim WH. A sirolimus: eluting stent fracture combined with a coronary artery aneurysm. Korean Circ J. 2008. 38:69–71.
7. Tizón-Marcos H, De Larochellière R, Larose E. Breakpoint: left main stent fracture: review of the literature. J Interv Cardiol. 2009. 22:362–367.
8. Adlakha S, Sheikh M, Bruhl S, et al. Coronary stent fracture: a cause of cardiac chest pain? Int J Cardiol. 2010. 141:e23–e25.
9. Bergelson BA, Tommaso CL. Left main coronary artery disease: assessment, diagnosis, and therapy. Am Heart J. 1995. 129:350–359.
10. Kim EJ, Rha SW, Wani SP, et al. Coronary stent fracture and restenosis in the drug-eluting stent era: do we have clues of management? Int J Cardiol. 2007. 120:417–419.
11. Liao R, Green NE, Chen SY, et al. Three-dimensional analysis of in vivo coronary stent: coronary artery interactions. Int J Cardiovasc Imaging. 2004. 20:305–313.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download