Abstract
We report our experience of very late stent thrombosis (VLST) in a young male patient who underwent implantation of two paclitaxel-eluting stents (PES) six years ago. The patient was compliant with standard dual antiplatelet therapy, but he presented with acute myocardial infarction which was associated with VLST. Intravascular ultrasound showed neointimal rupture with thrombus within the PES implanted in the right coronary artery. The lesion was successfully treated with balloon angioplasty without complications, however he was found to be hyporesponsive to clopidogrel when tested for adenosine diphosphate-induced platelet aggregation. The patient was discharged after uneventful recovery with triple anti-platelet therapy using aspirin, clopidogrel and cilostazol. To the best of our knowledge, a time interval of 2,223 days is the longest reported time interval between PES deployment and VLST occurrence. VLST may indeed occur in clinically stable patients, as multiple factors can influence the pathological mechanisms of VLST.
Drug-eluting stents (DES) provide clinical benefit by retarding smooth muscle cell replication and extracellular matrix production leading to restenosis, but may delay endothelial healing and heighten the risk of subsequent thrombosis.1)2) Although similar rates of early and late stent thrombosis were observed between DES and bare metal stent (BMS), a higher rate of very late stent thrombosis (VLST) beyond 12 months was reported with DES.3)
We present a case of very late paclitaxel-eluting stent (PES) thrombosis which occurred six years after successful implantation of DES.
A 39-year-old male underwent coronary stent implantation for stable angina pectoris in September 2004. The only risk factor included was dyslipidemia. The patient had a thrombotic nearly total occlusion of the middle right coronary artery (RCA) {Type C, 99%, thrombolysis in myocardial infarction (TIMI) flow I} (Fig. 1A) and a critical stenosis in the middle left anterior descending artery (LAD) (Type B2, 90%, TIMI flow III) (Fig. 1B). After intracoronary administration of a glycoprotein IIb/IIIa inhibitor (ReoPro®), percutaneous transluminal coronary angioplasty using a 3.5 mm balloon was performed, and a 3.5×32 mm PES (Taxus Express II stent, Boston Scientific Corporation Natick, MA, USA) was deployed in the RCA (Fig. 1C) and a 3.0×20 mm PES in the LAD (Fig. 1D). He was compliant with his medications which were as follows: aspirin 100 mg, clopidogrel 75 mg, cilostazol 200 mg, carvedilol 6.25 mg, losartan 25 mg, ezetimibe 10 mg, simvastatin 20 mg and isosorbide dinitrate 80 mg. In February 2005, follow-up coronary angiography was performed because of mild chest pain, and it showed no in-stent restenosis in both the coronary arteries (Fig. 2). Cilostazol was stopped 6 months after DES implantation. He was continued on medication with dual antiplatelet agents for 5 years. During follow-up, two-dimensional echocardiogram showed no regional wall motion abnormality with an ejection fraction of 69.4%, and the treadmill test was negative at 12.8 METS, just 3 months prior to re-admission.
The patient presented to the emergency room with sudden onset, left-sided chest pain without radiation in October 2010. A 12-lead ECG showed ST-segment elevation in the lead II, III, aVF and Mobitz type II second degree atrioventricular block (Fig. 3). He had a normal complete blood count, with white blood cell count of 5,900/mm3, hemoglobin of 13.8 g/dL, hematocrit of 41.1%, and platelet count of 199,000/mm3. The results of electrolyte panel, kidney function studies, liver function studies, and lipid panel were all within normal limits except for the low-density lipoprotein cholesterol of 86 mg/dL. Cardiac enzymes were initially normal.
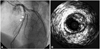
The patient underwent cardiac angiogram and it revealed stent thrombosis in the RCA (99%, I, 0) (Fig. 4A) and mild type II in-stent restenosis in the LAD (40%, III, 0) (Fig. 5). Intravascular ultrasound (IVUS) showed neointimal rupture with thrombus in the RCA stent (Fig. 4B). Plain old balloon angioplasty with a 3.5×15 mm balloon was performed in the RCA and the final coronary angiogram and IVUS showed good distal flow and markedly decreased residual stenosis in the RCA (Fig. 4C and D). He was found to be hyporesponsive to clopidogrel when tested for adenosine diphosphate-induced platelet aggregation utilizing the Verify Now P2Y12 point-of-care assay (301/14 P2Y12 reaction unit/%). He was discharged after uneventful recovery with triple anti-platelet therapy using aspirin 100 mg, clopidogrel 75 mg, cilostazol 200 mg daily. The patient has been followed-up at the outpatient department without any further symptoms.
The primary concern regarding long-term safety of DES is stent thrombosis, a potentially fatal adverse event that often leads to myocardial infarction or death. While randomized studies have not found an increased rate of late stent thrombosis in DES compared with BMS, reports of VLST in DES patients continue to increase in the literature with increasing use of these stents.4-8) VLST after the placement of a PES occurs in the range of 0.46-0.63%.9)10)
Although the mechanisms of VLST have not been completely understood, it is presumed that delayed endothelialization and chronic inflammation are implicated in the pathophysiology of VLST.11) Neointimal rupture is another possible mechanism underlying VLST development. Neointimal stent coverage has an effect on plaque stabilization due to the complete sealing of the stent by the neointima underlying the ruptured plaque. The exact mechanism of neointimal rupture remains unclear. Because the stent itself has thrombogenic potential, exposure of the stent through the ruptured neointima or due to inadequate neointima formation would increase the risk of thrombosis.12)13)
In the clinical setting, premature antiplatelet therapy discontinuation, renal failure, bifurcation lesions, diabetes and decreased left ventricular ejection fraction are the risk factors for stent thrombosis.14) Furthermore, non-responsiveness to clopidogrel is associated with higher risk of cardiovascular events, including cardiac death and stent thrombosis. The prevalence of clopidogrel resistance in the patient population was reported in the range of 5 to 44%.15)16) In this case, considering the fact that stent thrombosis occurred while he was on dual antiplatelet therapy and finally the patient was found to be hyporesponsive to clopidogrel, further investigations are needed to screen the patients at risk for stent thrombosis and to determine the adequate antiplatelet regimen beyond the standard dual antiplatelet therapy after DES implantation.
To the best of our knowledge, a time interval of 2,223 days is the longest reported time interval between PES deployment and VLST occurrence. We can conclude that VLST may indeed occur in clinically stable patients, as multiple factors can influence the pathological mechanisms of VLST. This case highlights the need for further long-term studies on VLST occurrence including the pathophysiology and predisposing factors of VLST, in patients treated with DES.
Figures and Tables
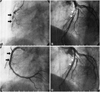 | Fig. 1Initial coronary angiography in September 2004. A: thrombotic nearly total occlusion of the middle right coronary artery (RCA) (Type C, 99%, TIMI flow I). B: critical stenosis in the middle left anterior descending artery (LAD) (Type B2, 90%, TIMI flow III). C: 3.5×32 mm paclitaxel-eluting stent (Taxus Express II stent) was deployed in the RCA. D: 3.0×20 mm paclitaxel-eluting stent in the LAD. The final coronary angiography showed good distal flow without residual stenosis in both the coronary arteries. TIMI: Thrombolysis in Myocardial Infarction. |
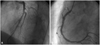 | Fig. 2Coronary angiography was performed 5 months later. No in-stent restenosis in the left anterior descending artery (A) and right coronary artery (B) stents were observed on follow-up coronary angiogram. |
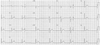 | Fig. 3A 12-lead electrocardiography showed ST-segment elevation in the lead II, III, aVF and Mobitz type II second degree atrioventricular block. |
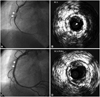 | Fig. 4A: right coronary angiogram showed very late stent thrombosis in the right coronary artery (RCA) stent. B: intravascular ultrasound (IVUS) showed neointimal rupture with thrombus within the RCA stent. C: coronary angiography after plain old balloon angioplasty. The final coronary angiogram showed good distal flow in the RCA. D: IVUS showed markedly decreased residual stenosis in the RCA. |
References
1. Farb A, Heller PF, Shroff S, et al. Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation. 2001. 104:473–479.
2. Rogers C, Parikh S, Seifert P, Edelman ER. Endogenous cell seeding: remnant endothelium after stenting enhances vascular repair. Circulation. 1996. 94:2909–2914.
3. Jensen LO, Maeng M, Kaltoft A, et al. Stent thrombosis, myocardial infarction, and death after drug-eluting and bare-metal stent coronary interventions. J Am Coll Cardiol. 2007. 50:463–470.
4. Seol SH, Kim DI, Han YC, et al. Multiple sequential complications after sirolimus-eluting stent implantation: very late stent thrombosis, stent fracture, in-stent restenosis, and peri-stent aneurysm. Korean Circ J. 2009. 39:439–442.
5. Kim SS, Jeong MH, Sim DS, et al. Very late thrombosis of a drug-eluting stent after discontinuation of dual antiplatelet therapy in a patient treated with both drug-eluting and bare-metal stents. Korean Circ J. 2009. 39:205–208.
6. Ahn YS, Cho JH, Kim DH, et al. A fatal case of simultaneous, very late thrombosis involving three drug-eluting stents in three coronary arteries. Korean Circ J. 2008. 38:564–569.
7. Nam CW, Kim KB, Chung IS. Very late stent thrombosis related to fracture of a sirolimus-eluting stent. Korean Circ J. 2007. 37:385–387.
8. Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007. 356:1020–1029.
9. Ellis SG, Colombo A, Grube E, et al. Incidence, timing, and correlates of stent thrombosis with the polymeric paclitaxel drug-eluting stent: a TAXUS II, IV, V, and VI meta-analysis of 3,445 patients followed for up to 3 years. J Am Coll Cardiol. 2007. 49:1043–1051.
10. Wenaweser P, Daemen J, Zwahlen M, et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol. 2008. 52:1134–1140.
11. Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006. 48:193–202.
12. Yokoyama S, Takano M, Sakai S, et al. Difference in neointimal proliferation between ruptured and non-ruptured segments after bare metal stent implantation. Int Heart J. 2010. 51:7–12.
13. Higo T, Ueda Y, Oyabu J, et al. Atherosclerotic and thrombogenic neointima formed over sirolimus drug-eluting stent: an angioscopic study. JACC Cardiovascular Imaging. 2009. 2:616–624.
14. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005. 293:2126–2130.
15. Müller I, Besta F, Schulz C, Massberg S, Schönig A, Gawaz M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemos. 2003. 89:783–787.
16. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Identification of low responders to a 300 mg clopidogrel loading dose in patients undergoing coronary stenting. Thromb Res. 2005. 115:101–108.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download