Abstract
Surgical skill and strategy for the correction of tetralogy of Fallot (TOF) have improved and resulted in satisfactory outcomes. However, prematurity and low birth weight continue to remain risk factors for poor outcomes. We present a case of a 2,150 g neonate born with TOF, in whom palliation was achieved with right ventricular outflow tract (RVOT) stenting. Seventy-seven days after the procedure, stenosis of RVOT below the stent was identified. At that time his body weight was 4.9 kg and total corrective surgery was deemed feasible. Eight months following surgical repair, the patient remained well without medical intervention. RVOT stenting may be a viable interim procedure while waiting for a low birth weight neonate born with TOF and prostaglandin E1 dependency to reach optimal weight to undergo corrective surgery.
Surgical skill and strategy for the correction of tetralogy of Fallot (TOF) have been improved and resulted in relatively satisfactory outcomes. However, prematurity and low birth weight continue to remain risk factors for poor outcomes.1) Consistent and optimal results have not been achieved in neonates who waited to reach adequate body weight for corrective surgery.2)3) Stenting of the right ventricular outflow track (RVOT) has been reported as a palliative procedure in low birth weight premature neonates.4-6)
We herein report a case of successful RVOT stenting in a low birth weight neonate born with TOF and prostaglandin E1 dependency. We think that this is the 1st Korean report of RVOT stenting.
A male neonate with a prenatal diagnosis of TOF associated with pulmonary atresia was born through vaginal delivery following 36 weeks and 5 days of gestation with 2,150 g body weight. Postnatal echocardiogram revealed TOF with severely restricted pulmonary flow secondary to infundibular hypertrophy. The pulmonary valve annulus diameter measured 3.0 mm. Diameters of branch pulmonary arteries measured 4.3 mm on the right and 4.2 mm on the left. There was a U-shaped patent ductus arteriosus that measured 2.4 mm in diameter. Because systemic oxygen saturation frequently fell below 60%, prostaglandin E1 could not be discontinued. Seven days after birth, cardiac catheterization for RVOT stenting was performed under general anesthesia. Cefamandole was administered prophylactically and a single dose of heparin was administered immediately following puncture of the right femoral vein. Hemodynamic studies and right ventriculography were performed in the conventional manner. We introduced a 5-F Multi-Purpose catheter (Cook Inc, Bloomington, USA) into the left pulmonary artery using a 0.032-inch Terumo wire (Terumo Corporation, Tokyo, Japan), which was exchanged for a 0.014 inch guide wire (Balance Middleweight, Abbott Vascular, Santa Clara, USA). After removing the 5-F catheter, a 7-F long sheath (SHUTTLE SL FLEXOR TUOHY BORST, Cook Inc, Bloomington, IL, USA) was introduced into the main pulmonary artery through a guide wire. A 5×15 mm stent (Palmaz Genesis amiia™, Cordis Corporation, Roden, Netherlands) was placed to extend from above the level of the pulmonary valve to the infundibulum. Because the proximal infundibulum was not covered by the stent, a 4×15 mm stent (Palmaz Genesis amiia™, Cordis Corporation, Roden, Netherlands) was additionally inserted to fully cover the infundibulum (Fig. 1). Neither arrhythmia nor conduction disturbance occurred secondary to the procedure. Total fluoroscopy time was 25 minutes. Oxygen saturation of arterial blood was increased to 92% following stent insertion, at which point prostaglandin E1 was deemed safe for discontinuation. Six days following stent insertion, the patient was discharged with aspirin at 5 mg/kg/d. Seventy-seven days following stent insertion, the infant developed significant cyanosis. His percutaneous oxygen saturation dropped to 68% during irritable state. Echocardiography measured peak velocity at 4.3 m/s in the RVOT and stenosis of the RVOT below the stent was suspected. The diameter of the main pulmonary artery measured 5.0 mm and the diameters of the branch pulmonary arteries measured 5.3 mm on the right, and 6.4 mm on the left. At this time his body weight was 4.9 kg and total corrective surgery was deemed appropriate. Intraoperatively, stenosis of the RVOT below the stent was confirmed. The ventricular septal defect was closed with a Dacron patch and RVOT widening was performed by infundibulectomy and transannular autologous pericardial patch insertion following stent removal. The infant was discharged 25 days after surgery. Eight months following corrective operation he remained well without medical intervention.
After the initial echocardiographic examination of this patient, we did not consider waiting for his body weight to be optimal for the procedure. Also, we discontinued infusion of prostaglandin E1, because it has been associated with higher mortality and morbidity.2)3) Early neonatal correction of TOF has been supported by some clinicians.7) According to the 'early repair' strategy, cardiopulmonary bypass can be performed in infants weighing as little as 2 kg.7) Therefore, our patient might have been a candidate for 'early repair'. However, this strategy remains controversial7)8) and is not preferred at our institution.
Palliative methods for TOF include systemic to pulmonary arterial shunt insertion, particularly the modified Blalock-Taussig shunt, stenting of ductus arteriosus, balloon pulmonary valvuloplasty and RVOT stenting. Pulmonary artery hypoplasia and distortion are common complications following initial palliation by modified Blalock-Taussig shunt,9) particularly when palliation was performed during the neonatal period.10) According to Gladman et al.10) neonatal palliation by modified Blalock-Taussig shunt was associated with moderate to severe distortion of the right pulmonary artery in 35% of patients, and the need for pulmonary artery intervention in 60% of patients. Therefore, we excluded the modified Blalock-Taussig shunt as initial palliation. In addition, because the ductus arteriosus of our patient was 'U' shaped, we excluded its stenting as an option, and severe infundibular hypertrophy excluded isolated balloon pulmonary valvuloplasty11) as a selective palliative method. We ultimately decided to insert a RVOT stent. He was discharged following the procedure, and gained adequate body weight for corrective repair of TOF.
Dohlen et al.4) reported that utilization of low profile, flexible pre-mounted coronary stent had greatly simplified their practice of RVOT stenting. We decided not to use it, because medical insurance does not cover use of coronary stent for stenting non-coronary vessels in the Republic of Korea. The optimal diameter of RVOT stent suggested by Dohlen et al.4) is 1-2 mm larger than the infundibular diameter during diastole, and the minimal size of stents used in their study was 4 mm. The length of the stent should be enough to cover the entire stenotic RVOT, and careful attention is needed in the positioning.
Two challenges arose in our case. First, two stents were inserted to cover the infundibulum. Second, we were only able to defer performing corrective surgery by 3 months. The 3 months gained appeared to be sufficient to overcome complications associated with repair during early infancy,12) but was a relatively short period to perform 'right ventricular infundibulum sparing' strategy.8) In conclusion, the potential of growth of proximal infundibulum should be taken into account when considering stent positioning.
Figures and Tables
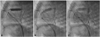 | Fig. 1A: pulmonary angiogram showing insertion of 1st stent into the pulmonary value to the infundibulum via main pulmonary artery. B: but the proximal infundibulum was not covered by stent. Another stent additionally inserted for full covering of infundibulum. C: pulmonary angiogram showing insertion of two stents. |
References
1. Wernovsky G, Rubenstein SD, Spray TL. Cardiac surgery in the low-birth weight neonate: new approaches. Clin Perinatol. 2001. 28:249–264.
2. Chang AC, Hanley FL, Lock JE, Castaneda AR, Wessel DL. Management and outcome of low birth weight neonates with congenital heart disease. J Pediatr. 1994. 124:461–466.
3. Reddy VM, McElhinney DB, Sagrado T, Parry AJ, Teitel DF, Hanley FL. Results of 102 cases of complete repair of congenital heart defects in patients weighing 700 to 2500 grams. J Thorac Cardiovasc Surg. 1999. 117:324–331.
4. Dohlen G, Chaturvedi RR, Benson LN, et al. Stenting of the right ventricular outflow tract in the symptomatic infant with tetralogy of Fallot. Heart. 2009. 95:142–147.
5. Gibbs JL, Uzun O, Blackburn ME, Parsons JM, Dickinson DF. Right ventricular outflow stent implantation: an alternative to palliative surgical relief of infundibular pulmonary stenosis. Heart. 1997. 77:176–179.
6. Laudito A, Bandisode VM, Lucas JF, Radtke WA, Adamson WT, Bradley SM. Right ventricular outflow tract stent as a bridge to surgery in a premature infant with tetralogy of Fallot. Ann Thorac Surg. 2006. 81:744–746.
7. Jonas RA. Early primary repair of tetralogy of Fallot. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2009. 39–47.
8. Morales DL, Zafar F, Fraser CD Jr. Tetralogy of Fallot repair: the right ventricle infundibulum sparing (RVIS) strategy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2009. 12:54–58.
9. Lee Y, Yang KM, Rho JR, Lee YK. Palliative surgery for tetralogy of Fallot: report of 43 cases. Korean Circ J. 1971. 1:17–21.
10. Gladman G, McCrindle BW, Williams WG, Freedom RM, Benson LN. The modified blalock-taussig shunt: clinical impact and morbidity in Fallot's tetralogy in the current era. J Thorac Cardiovasc Surg. 1997. 114:25–30.
11. Song JY, Yoo JJ, Noh JI, Choi JY, Yoon YS. Tentative results of balloon dilatation of the pulmonary valves. Korean Circ J. 2000. 30:745–750.
12. Van Arsdell GS, Maharaj GS, Tom J, et al. What is the optimal age for repair of tetralogy of Fallot? Circulation. 2000. 102:19 Suppl 3. III123–III129.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download