Abstract
We present an unusual case of a delayed right ventricular perforation by a single standard-caliber implantable cardioverter-defibrillator lead, which manifested 14 days after implantation. Multidetector computed tomography could clearly display the lead perforation, and allow for identification of the associated sequelae such as pericardial effusion and planning the lead extraction strategy.
Severe complications have been observed more frequently in transvenous implantation of implantable cardioverter-defibrillator (ICD) lead than in that of pacemaker, particularly in the dual-chamber rather than single-chamber ICD.1)2) Also, perforation of the right ventricular (RV) wall is a rare complication in ICD lead implantation with an estimated incidence of 0.6%. This complication mostly occurs in the perioperative period and low incidence of delayed RV wall perforation in the subacute phase has been reported.3) Detection of RV wall perforation by standard imaging modalities is often challenging. Multidetector computed tomography (MDCT) is emerging as an optional modality for establishing an accurate diagnosis of lead perforation, identifying the extent of accompanying complications such as pericardial effusion and planning the lead extraction strategy. We present a rare case of delayed RV perforation that manifested 14 days after ICD implantation and was clearly displayed by MDCT.
An 81-year-old man was referred to our hospital for presyncope, dyspnea and palpitation. He had a previous history of inferoposterior myocardial infarction treated by percutaneous coronary intervention with drug-eluting stents (DES). On arrival, he was conscious and alert but his heart rate was 190 beats/min and the electrocardiogram (ECG) revealed sustained ventricular tachycardia. Intravenous administration of adenosine or lidocaine did not stabilize the rhythm, and electrical cardioversion finally restored a normal sinus rhythm. After the recovery of sinus rhythm, the ECG did not show ischemic ST-T changes and serum creatine kinase as well as troponin T levels were not elevated. The echocardiography revealed left ventricular (LV) dilatation with LV end-diastolic/end-systolic dimensions of 66/57 mm, decreased LV ejection fraction of 35% and severe ischemic mitral regurgitation. Coronary angiography did not demonstrate any significant change compared to the previous findings. The implantation of ICD with an atrial passive-fixation lead (model 5076, CapSureFix, Medtronic, Minneapolis, MN, USA) and a standard-caliber ventricular active-fixation lead (model 6947, Sprint Quattro 8.6-French lead, Medtronic, Minneapolis, MN, USA) was performed through the left subclavian vein without any complications. The two leads were placed in the right atrial appendage and right ventricular apex, respectively (Fig. 1A and B). The RV lead parameters were satisfactory (R-wave amplitude 6.9 mV, pacing threshold 0.4 V at 0.5 ms, pacing impedance 380 ohms). After the procedure, dual anti-platelet therapy (aspirin 100 mg and ticlopidine 200 mg per day) was commenced immediately because of DES implantation 9 months ago. Echocardiography performed on post-implant day 7 showed no significant change without any evidence of pericardial effusion.
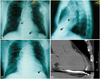
Fourteen days after ICD implantation, the patient presented to the emergency room with intermittent chest pain since the last 2 days. The chest pain appeared to be independent of exercise and lasted for 10 to 20 seconds. He was hemodynamically stable. The ECG as well as circulatory biomarker measurements such as creatine kinase and troponin T indicated no signs of acute coronary syndrome. The chest X-ray showed cardiomegaly and minor changes in the ventricular lead tension and ventricular lead tip position (Fig. 1C). The echocardiography revealed moderate pericardial effusion without any significant change in LV wall motion. Interrogation of the ICD showed that the sensed R wave measured 6.0 mV, the ventricular pacing threshold had risen to 8 V at 0.5 ms and pacing impedance had dropped to 282 ohms. Thus, RV perforation by the ICD lead was suspected and 64-slice MDCT was subsequently performed with a MSCT scanner (VCT, GE Healthcare, Milwaukee, WI, USA) according to the following protocol: 120 kV, 300 mA, rotation time of 350 to 400 msec and collimation of 64×0.625 mm but without contrast-media to avoid further deterioration of renal function (Fig. 1D). MDCT confirmed complete lead perforation, and because of the high bleeding risk due to dual anti-platelet therapy, cardiac surgery under general anesthesia rather than manual lead extraction was indicated (Fig. 2). Lead extraction, closure of RV perforation site and re-implantation of the RV lead onto the RV outflow tract were successfully performed. The patient had an uneventful recovery and has been doing well since the last 6 months after discharge.
MDCT allows ready identification of the course of the lead, relationship to adjacent anatomy and adverse sequelae such as pericardial effusion.4) This case highlights the role of MDCT as an extremely useful imaging modality to confirm the diagnosis of lead perforation and planning the lead extraction strategy, even without contrast enhancement.
The precise mechanism responsible for delayed lead perforation remains unclear. Mechanisms responsible for RV lead perforation generally involve lead-related factors such as the stiffness and diameter of the lead as well as myocardium-related factors such as morphology of the right ventricular wall and contraction during each heart beat.5) Patients with thinning of the ventricular wall are at a higher risk of lead perforation. Several reports have demonstrated that delayed lead-related complications such as lead perforation and lead failures were more frequent with the use of small-caliber ICD leads, especially the St. Jude Riata leads,6) than with standard-caliber ICD leads,7) thereby suggesting the important contribution of lead-related factors. In the present case, thinning of RV wall due to an old myocardial infarction caused by occlusion of the proximal right coronary artery might have critically contributed to the occurrence of lead perforation, since the standard-caliber active fixation lead (model 6947, Sprint Quattro 8.6-French lead, Medtronic) was implanted at the RV apex. The incidence of ICD lead perforation has been reported to be approximately 0.5% in two independent studies including 593 and 3,340 cases, respectively.8)9) As previous reports have shown, lead perforation commonly occurred within 7 to 10 days after implantation, mostly occurring at the time of implantation or within the first 24 hours.6)8) In addition, small-caliber ICD leads were used in many of the recently reported cases of lead perforation. Given the type of implanted ICD leads used and the timing of RV perforation, this is a rare case of ICD lead perforation clearly displayed by MDCT.
With respect to the treatment of lead perforation, transvenous lead extraction under transesophageal echocardiographic monitoring might be a standard procedure, in case of active-fixation lead.1) However, we considered the present case to be at a high risk for hemodynamic and/or uncontrollable bleeding complications, because of decreased LV function with severe mitral regurgitation and dual anti-platelet therapy. Thus, we selected open surgery via median sternotomy as the safest approach.
Operators of ICD implantation should always keep in mind that pericardial effusion due to lead perforation may develop without the typical manifestations of cardiac tamponade in the chronic phase.
Figures and Tables
Fig. 1
Chest X-rays in the postero-anterior view (A) and lateral view (B) indicating positions of the atrial and defibrillator leads immediately after initial implantation. The defibrillator lead tip (solid arrow) was positioned within the right ventricular apex and the atrial lead tip (dashed arrow) was positioned within the right atrial appendage. (C) Chest X-ray 14 days after initial implantation in the emergency department showing cardiomegaly and minor changes in ventricular lead tension and ventricular lead tip position (solid arrow). (D) Multidetector computed tomography scan of the thorax showing cardiac perforation by defibrillator lead. The defibrillator lead tip (white arrow), identifiable by its radiological density and associated scatter artifacts, extends outwards beyond the right ventricular myocardium into the pericardial sac.
References
1. Polin GM, Zado E, Nayak H, et al. Proper management of pericardial tamponade as a late complication of implantable cardiac device placement. Am J Cardiol. 2006. 98:223–225.
2. Takahashi T, Bhandari AK, Watanuki M, Cannom DS, Sakurada H, Hiraoka M. High incidence of device-related and lead-related complications in the dual-chamber implantable cardioverter defibrillator compared with the single-chamber version. Circ J. 2002. 66:746–750.
3. Schwartzman D, Nallamothu N, Callans DJ, Preminger MW, Gottlieb CD, Marchlinski FE. Postoperative lead-related complications in patients with nonthoracotomy defibrillation lead systems. J Am Coll Cardiol. 1995. 26:776–786.
4. Henrikson CA, Leng CT, Yuh DD, Brinker JA. Computed tomography to assess possible cardiac lead perforation. Pacing Clin Electrophysiol. 2006. 29:509–511.
5. Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol. 2007. 30:28–32.
6. Danik SB, Mansour M, Singh J, et al. Increased incidence of subacute lead perforation noted with one implantable cardioverter-defibrillator. Heart Rhythm. 2007. 4:439–442.
7. Ellis CR, Rottman JN. Increased rate of subacute lead complications with small-caliber implantable cardioverter-defibrillator leads. Heart Rhythm. 2009. 6:619–624.
8. Turakhia M, Prasad M, Olgin J, et al. Rates and severity of perforation from implantable cardioverter-defibrillator leads: a 4-year study. J Interv Card Electrophysiol. 2009. 24:47–52.
9. Lee DS, Krahn AD, Healey JS, et al. Evaluation of early complications related to De Novo cardioverter defibrillator implantation insights from the Ontario ICD database. J Am Coll Cardiol. 2010. 55:774–782.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download