Abstract
Coronary artery bypass graft (CABG) intervention, particularly anastomosis site intervention, is challenging for interventional cardiologists. A paclitaxel-eluting balloon catheter (SeQuent Please) is a recently-introduced device capable of delivering paclitaxel homogeneously into the targeted vessel wall. We herein report our experience with two cases. In the first case, coronary angiography showed significant stenosis at the site of anastomosis between the saphenous vein graft and the left anterior descending artery (LAD). In the second case, coronary angiography showed significant stenosis at the site of anastomosis between the left internal mammary artery and the LAD. We performed percutaneous intervention of these CABG anastomoses using paclitaxel-eluting balloon catheters, and obtained favorable angiographic and clinical outcomes.
Coronary artery bypass surgery is the treatment of choice in patients with left main coronary artery disease or triple-vessel disease.1)2) However, graft occlusion remains a challenge for interventional cardiologists, particularly in patients who develop occlusion at the site of anastomosis. Recently, a paclitaxel-eluting balloon catheter (SeQuent Please, B. Braun, Melsungen, Germany) was introduced and showed favorable results in the management of in-stent restenosis, small vessel disease, and bifurcation lesions.3-5) We herein report two cases of percutaneous intervention for the management of coronary artery bypass graft (CABG) anastomosis using paclitaxel-eluting balloon catheters.
A 77 year-old male patient was admitted through the outpatient clinic with a 1-month history of chest pain on exertion. His medical history included hypertension for 15 years. He underwent coronary artery bypass surgery for myocardial infarction 5 years previously. A saphenous vein graft (SVG) was anastomosed with the ascending aorta and the distal left anterior descending artery (LAD). Post-operatively, he had been taking aspirin, clopidogrel, atorvastatin, and nitrates. Five months prior to his presentation, percutaneous balloon angioplasty was performed with a 3.0×20 mm Ryujin balloon (Terumo, Tokyo, Japan) at 10 atmospheres because coronary angiography showed significant stenosis of the anastomosis between the SVG and the distal LAD. On admission, his blood pressure and pulse rate were 110/76 mm Hg and 53/min, respectively. Electrocardiography (ECG) showed sinus bradycardia, left ventricular hypertrophy with strain, and Q waves in leads III and aVF. The levels of creatinine kinase-MB (CK-MB) and troponin-I were within the normal limits. Echocardiography demonstrated an ejection fraction of 54% and hypokinesia of the inferior wall. Left coronary angiography showed significant stenosis of the left main coronary artery, total occlusion of the proximal LAD and the proximal left circumflex artery (LCx). Right coronary angiography showed significant diffuse stenosis from the proximal-to-distal right coronary artery (RCA). SVG angiography showed significant restenosis of the anastomosis between the SVG and the distal LAD, and significant stenosis in the distal LAD. We decided to perform on-site percutaneous intervention of the anastomosis. A 6-Fr Judkins right guiding catheter (Cordis, Johnson & Johnson, Bridgewater, NJ, USA) was engaged in the SVG through the right femoral artery. A 0.014-inch Fielder FC wire (Ashahi Intecc, Nagoya, Japan) was placed in the SVG to the LAD. We performed angioplasty with a 2.5×15 mm Ryujin balloon (Terumo) at 8 atmospheres, and a 2.75×15 mm SeQuent Please paclitaxel-eluting balloon catheter (B. Braun, Melsungen, Germany) at 7 atmospheres. Post-intervention angiography showed minimal residual stenosis and Thrombolysis in Myocardial Infarction (TIMI) grade III blood flow. Four days after the intervention, he was discharged without chest pain. Four months later, repeat coronary angiography showed no restenosis at the site of the anastomosis (Fig. 1).
A 71-year-old female patient was admitted through the outpatient clinic with a 3-month history of chest pain on exertion. Her past medical history included hypertension for 12 years. She underwent coronary artery bypass surgery for myocardial infarction secondary to triple-vessel disease 2 years prior to admission. She was managed by anastomosing the left internal mammary artery (LIMA) with the distal LAD. Two SVGs were anastomosed with the obtuse marginal branch and distal RCA. Post-operatively, she was commenced on aspirin, clopidogrel, pitavastatin, and nitrates. On admission, her blood pressure and pulse rate were 124/70 mm Hg and 75/min, respectively. ECG showed normal sinus rhythm. Levels of CK-MB and troponin-I were within the normal limits. Echocardiography showed an ejection fraction of 53% and hypokinesia of the inferior wall. Left coronary angiography showed total occlusion of the proximal LAD and diffuse stenosis of the proximal LCx. Right coronary angiography showed diffuse stenosis of the proximal-to-mid RCA and total occlusion of the distal RCA. LIMA angiography showed significant stenoses of the mid LAD and anastomosis between the LIMA and the distal LAD. SVG angiography showed two patent SVGs. The following day she underwent myocardial perfusion scan, which confirmed perfusion defect of the anteroseptal wall in the adenosine stress phase. We considered mid LAD stenosis, and the anastomosis between the LIMA and LAD as the cause of her clinical state, and decided to perform percutaneous intervention at these sites. A 6-Fr Judkins right guiding catheter (Cordis) was engaged in the SVG through the right femoral artery. A 0.014 inch Fielder FC wire (Ashahi Intecc) was placed in the LIMA to mid LAD. We performed angioplasty of the anastomosis with a 2.0×15 mm Maverick balloon (Boston Scientific, Natick, MA, USA) at 6 atmospheres. We attempted to deliver a drug-eluting stent into the lesion, but it would not advance beyond a site just before the lesion. Therefore, we replaced a drug-eluting stent with a 2.5×17-mm SeQuent Please paclitaxel-eluting balloon catheter (B. Braun) at 7 atmospheres. We performed angioplasty of the mid LAD with a 2.0×15 mm Maverick balloon (Boston Scientific) at 8 atmospheres. Post-intervention angiography showed minimal residual stenosis and blood flow of TIMI grade III. The patient was discharged three days after the intervention without chest pain. Six months later, coronary angiography showed no restenosis of the anastomosis (Fig. 2).
Graft occlusion can be troublesome in patients who have undergone coronary artery bypass surgery. Patency rates depend on bypass conduits. Patency (stenosis <20%) rates of a LIMA at 5 and 10 years have been reported to be 98% and 95%, respectively, while the patency rates of a SVG are 95% and 71%, respectively.6) Patency rate of a radial artery graft at 5 years is 95% in younger patients, (<70 years) and 86% in older patients (≥70 years).7)
Treatment options of graft occlusion include percutaneous balloon angioplasty, percutaneous stenting, and redo-CABG. Because redo-CABG is associated with high mortality rate,8) percutaneous intervention is preferred. Because cardiac surgeons have been using SVG for a long time, and because SVG has a relatively low patency rate, interventional cardiologists should occasionally perform SVG intervention. However, SVG intervention is challenging because of the high rate of periprocedural myocardial infarction and restenosis.9) In addition, a stent in the SVG can fracture due to movement of the SVG.10) Recent reports have demonstrated that drug-eluting stents and embolic protection devices lead to good results in the management of SVG disease.11)12) Because a LIMA graft has high patency rate, LIMA intervention is not performed frequently. For LIMA intervention, favorable results can be obtained by stenting.13)
Intervention of distal anastomosis is more challenging, because distal anastomosis is bifurcating, and different vessel response to intraluminal ballooning exists between the graft and native coronary artery. Standard treatment modalities of distal anastomosis disease have not been established.
The recently-released paclitaxel-eluting balloon catheter, which is based on a new matrix-coating technique, is polymer-free and can deliver paclitaxel homogeneously into the targeted vessel wall. Because there are no polymers on the paclitaxel-eluting balloon, the risk of intracoronary thrombosis is lower than that with a drug-eluting stent. Therefore, the need for long-term dual anti-platelet therapy should be reduced. According to experienced operators, deliverability of a paclitaxel-eluting balloon is better than a drug-eluting stent. It has been reported that paclitaxel-eluting balloon catheters can be a treatment option of in-stent restenosis, small vessel disease, and bifurcating lesions.3-5) In the PEPCAD II trial, paclitaxel-eluting balloons had comparable binary restenosis rate to paclitaxel-eluting stents for the treatment of coronary in-stent restenosis at 6-month follow-up.14) However, in the PICCOLETO study, paclitaxel-eluting balloon catheters failed to show superior angiographic and clinical outcomes for the intervention of small coronary arteries compared to paclitaxel-eluting stents.15) The potential use and long-term restenosis rate of paclitaxel-eluting balloon catheters have not been studied.
We successfully managed two cases of percutaneous intervention of distal anastomoses of CABGs with paclitaxel-eluting balloon catheters. Short-term angiographic and clinical outcomes were favorable. In the cases, because of difference in the proximal and distal reference diameters and because of the acute angles between the proximal and distal arteries, risks of stent mal-apposition and fracture is high following stenting of the anastomosis sites. Therefore, paclitaxel-eluting balloons were better choices than conventional stents. Because of good deliverability and comparable restenosis rate,3-5) intervention with a paclitaxel-eluting balloon catheter can be an option for the treatment of CABG distal anastomosis. Long-term randomized studies are required to evaluate paclitaxel-eluting balloon catheters for the intervention of CABG.
Figures and Tables
Fig. 1
A: saphenous vein graft (SVG) angiography of the patient in Case 1 showed significant restenosis of anastomosis between the SVG and the distal left anterior descending artery (LAD), and significant stenosis in the distal LAD. B: post-intervention angiography showed minimal residual stenosis and blood flow of Thrombolysis in Myocardial Infarction (TIMI) grade III. C: four-month follow-up angiography showed no restenosis and blood flow of TIMI grade III.
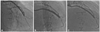
Fig. 2
A: left internal mammary artery (LIMA) angiography of patient in Case 2 showed significant stenosis of the mid left anterior descending artery (LAD) and anastomosis between the LIMA and the distal LAD. B: post-intervention angiography showed minimal residual stenosis and blood flow of Thrombolysis in Myocardial Infarction (TIMI) grade III. C: six-month follow-up angiography showed no restenosis and blood flow of TIMI grade III.

References
1. Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009. 120:2271–2306.
2. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007. 116:e148–e304. Abstract.
3. Scheller B, Hehrlein C, Bocksch W, et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006. 355:2113–2124.
4. Unverdorben M, Kleber FX, Heuer H, et al. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2010. 99:165–174.
5. Fanggiday JC, Stella PR, Guyomi SH, Doevendans PA. Safety and efficacy of drug-eluting balloons in percutaneous treatment of bifurcation lesions: the DEBIUT (drug-eluting balloon in bifurcation Utrecht) registry. Catheter Cardiovasc Interv. 2008. 71:629–635.
6. Tatoulis J, Buxton BF, Fuller JA. Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg. 2004. 77:93–101.
7. Buxton BF, Raman JS, Ruengsakulrach P, et al. Radial artery patency and clinical outcomes: five-year interim results of a randomized trial. J Thorac Cardiovasc Surg. 2003. 125:1363–1371.
8. Weintraub WS, Jones EL, Craver JM, Grosswald R, Guyton RA. In-hospital and long-term outcome after reoperative coronary artery bypass graft surgery. Circulation. 1995. 92:9 Suppl. II50–II57.
9. Keeley EC, Velez CA, O'Neill WW, Safian RD. Long-term clinical outcomes and predictors of major adverse cardiac events after percutaneous interventions on saphenous vein grafts. J Am Coll Cardiol. 2001. 38:659–665.
10. Yoo SH, Jin SW, Her SH, et al. Complete fracture of sirolimus-eluting stent in a saphenous vein graft to left anterior descending artery. Korean Circ J. 2009. 39:251–253.
11. Lee MS, Yang T, Kandzari DE, Tobis JM, Liao H, Mahmud E. Comparison by meta-analysis of drug-eluting stents and bare metal stents for saphenous vein graft intervention. Am J Cardiol. 2010. 105:1076–1082.
12. Hiscock M, Oqueli E, Dick R. Percutaneous saphenous vein graft intervention: a review. Heart Lung Circ. 2007. 16:Suppl 3. S51–S55.
13. Morrison DA, Thai H, Goldman S, Felix E, Hernandez J. Percutaneous coronary intervention of or through saphenous vein grafts or internal mammary arteries: the impact of stents, adjunctive pharmacology, and multicomponent distal protection. Catheter Cardiovasc Interv. 2006. 67:571–579.
14. Unverdorben M, Vallbracht C, Cremers B, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. 2009. 119:2986–2994.
15. Cortese B, Micheli A, Picchi A, et al. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospec-tive randomized clinical trial: the PICCOLETO Study. Heart. 2010. 96:1291–1296.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download