Abstract
Atrial septal defect (ASD) is the most common type of common congenital heart disease (CHD) in adults. During the last decade, there has been a remarkable change in the treatment strategy of ASD, shifting the therapeutic gold standard from surgery to transcatheter closure, along with refinements and the evolution of device technology. Reports on the outcome of transcatheter ASD closure have shown an excellent efficacy as well as a low complication rate. However, the procedural details and/or outcomes of this procedure may be influenced by several factors including morphologic characteristics of the defect, co-morbid diseases, as well as individual factors including age and weight of the patient. Because the risk-benefit relationship in both the very young and the elderly subsets of the patients has not been clearly defined yet, closure of an ASD with device may be potentially subtracted from the treatment option in these patient groups. In this article, we will review the basis for device closure in small children and elderly patients with ASD and provide an overview of the frequently encountered problems.
Atrial septal defect (ASD) is a common congenital heart disease (CHD) which accounts for 8-10% of all CHD and is considered to be the most common type of CHD in adults. Among various types of ASD, the ostium secundum defect exceeds other types in prevalence and is a good candidate for device closure. The therapeutic strategy for secundum ASD has shown a remarkable change in the last decade because of the evolution of device technology, and currently transcatheter closure has become the primary treatment option for most patients in many centers. The reported outcomes of transcatheter ASD closure have been excellent with low complication rates. On the other hand, this "usually straightforward" procedure has several influencing factors that play a role in the procedural details and/or outcomes, such as morphologic characteristics of the defect, co-morbid disease, as well as individual factors including age and weight of the patient. Because the risk-benefit relationship in both the very young and the elderly groups of patients has not been clearly defined yet, closure of an ASD with device may be potentially subtracted from the treatment option in these patient groups.
Patients with isolated ASD are usually asymptomatic through infancy and early childhood and the elective surgery may be deferred to 2-4 years of age.1) However, it has also been well documented that substantial numbers of small children are in need of earlier intervention, especially when the disease is complicated by chronic lung disease in prematurely-born infants or associated with certain chromosomal anomalies.2)3) Nevertheless, the need for early treatment has been recognized in small children who are showing rapidly enlarging defects to prevent rendering a suitable ASD to an unsuitable ASD for device closure.4)5) Some authors advocate that any defect larger than 8 mm with evidence of a significant left to right shunt should be closed even in very young patients, because such a defect will likely never close spontaneously and may even get larger.6) Indeed, in a study on the change in size of ASD, a significant enlargement of the defect, more than 50% of the initial size, has been detected in 30% of the studied population, which suggests that there is a potential for some of the defects to outgrow a possible transcatheter closure.7)
Recent articles on surgical treatment of ASD in young and symptomatic infants emphasize on benefits of early surgery on the grounds of a dramatically improved clinical course.3) There has been a study reporting on increased procedural time and lower procedural success rate for transcatheter ASD closure during their early experience with the Amplatzer septal occluder (ASO, AGA medical corporation, Plymouth, MN, USA) in symptomatic small children.8) However, another recent report states that device closure is a safer and more effective alternative to surgery, with valid advantages in very small children, including infants and those who weigh less than 10 kg.9-12) Cardenas et al.10) reported that there was no additional risk in smaller babies (<10 kg) for transcatheter ASD closure, and also in our study comparing outcomes of device therapy to those of surgical closure in small children with 10 kg of weight or less, the superior safety of the device group was evident, which was proved by a lower complication rate.11)
There are several problems encountered during device closure in small children. The vessels are relatively small compared to the large delivery system, which leaves the possibility for potential damage to vascular access. Moreover, because of the rigid coupling mechanism between the device and delivery cable as well as the relatively stiff delivery system, there is a latent risk for damage to the cardiac tissue. The inherent design of ASO with the relatively-excessive rim width of atrial discs in smaller devices (Fig. 1) may also increase the potential risk for erosion or cessation of the procedure, because the excessive atrial rims may encroach on the adjacent cardiac structures or atrioventricular valves. Because of the small total septal length in small hearts, plans for implantation of large devices may occasionally be thwarted, especially in small children with relative mitral rim deficiency. Therefore, in order to ensure procedural safety, special care must be taken to prevent damage to the vasculature and to cardiac structures, and the procedure should be conducted by an experienced hand in this subset of the patient population. Also, further modifications of the design for smaller sizes of the device to fit a small heart may be needed to increase the applicability of the procedure to smaller children. On the other hand, a "stopgap technique", which is choosing the device size according to the size of the heart so the left atrial disc diameter can fit the maximal septal length has been suggested.13) This technique may be a good solution for small children with a relatively large defect diameter to total septal length ratio, but the interpretation for the usefulness of this technique still requires further investigation.
Elderly patients with hemodynamically significant ASD frequently experience complications with serious long-term adverse consequences such as pulmonary hypertension, atrial arrhythmia, and atrioventricular valve insufficiencies that essentially involve the tricuspid valve as well as right-sided heart failure secondary to chronic volume overload of the right side of the heart.14) This group of patients has a higher prevalence of co-morbid cardiovascular and/or systemic diseases including systemic hypertension, stroke, chronic lung disease, diabetes mellitus, atherosclerosis and coronary heart disease.14-16) The pathophysiologic mechanism is summarized in Fig. 2. Longstanding left to right shunt in the atrial level results in progressive right heart dilatation, significant tricuspid insufficiency and subsequent increase in right atrial pressure. Left heart may also be influenced by chronic volume underload, increased atrial pressure as well as co-morbid diseases such as systemic hypertension or coronary heart disease. Significant numbers of the patients are also affected by more advanced complications inherent to the end stage of this pathophysiologic cascade, including pulmonary hypertension, ventricular dysfunction and atrial arrhythmias which cause significant symptoms. In addition, diastolic compression of left ventricle (LV) by dilated right ventricle reduces LV end-diastolic volume in chronic setting, and this is responsible for so called "masked LV restriction" which may cause serious pulmonary edema secondary to LV dysfunction and left atrium (LA) pressure increase after ASD closure.17) Because of the chronic nature of the disease, patients are usually adapted to the disabilities they have had and invasive interventions are occasionally refused by patients. Nevertheless, scarce evidence for survival benefit of anatomical closure and higher potential risk of definitive treatment in this group of patients may sometimes affect the decision to a more conservative one.
The benefits from ASD closure in the elderly population has been documented in fewer reports compared to the younger population. In the 1990s, there was a nonrandomized study debating the clinical benefits between medical treatment only and anatomical closure.18) However, a relatively recent randomized study in a large population confirmed that anatomical closure is superior to medical treatment in preventing major events.19) Moreover, the realistic benefits of ASD closure include symptomatic relief, improvements of functional status as well as the quality of life.20)21) These favorable clinical changes are supported by the immediate and substantial reverse remodeling of the heart after closure from many studies.14)16)17)22) Excellent safety and efficacy in terms of improving functional class and favorable reverse remodeling of the heart have been consistently reported, and the solutions for major complications such as restrictive LV, chronic atrial arrhythmia and pulmonary hypertension have been discussed as major issues.
The most important problem in elderly patients is that they have more complex presentations of the disease, mainly because they frequently have co-morbid systemic and heart diseases as previously mentioned. This necessitates precise evaluation of the risk factors as an essential part of successful treatment. Co-morbid diseases in elderly patients in recent publications are shown on Table 1.14-16) More than one third of the patients showed systemic hypertension and systemic diseases such as diabetes mellitus, and a considerable extent of pulmonary and neurological diseases were also present.
Cardiac co-morbidities include pulmonary hypertension in nearly half of the patients, chronic atrial arrhythmia in more than 20% and ischemic heart disease in about 15% of the patients. Post-closure pulmonary edema caused by "masked LV restriction" may appear in 2 to 4% of the elderly patients and should be ruled out before ASD closure through a balloon occlusion test. It was previously described that most of the patients with evidence of LV restriction during temporary balloon occlusion of the defect may show favorable response to several days of anti-congestive therapy, so that they could go through with the device closure without any serious events.
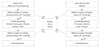
Even though LV dysfunction after ASD closure is not a frequent finding, it may lead to a serious consequence once it occurs. Thus, the detection of the risk for serious LV restriction before the procedure is very important. There are 2 different protocols for detection and management of high risk patients, which have been independently developed by 2 separate groups (Fig. 3).5)14)15)17)23) Both protocols involve a temporary balloon occlusion test and measurement of LA pressure, but the definition of significant LV restriction is quite different for each protocol. Both protocols recommend several days of medical therapy including diuretics, inotropic agents and afterload reducing therapy once LV restriction is diagnosed, and if the patient proves to be refractory to medical therapy on a subsequent balloon occlusion test, they are recommended a fenestrated device (Fig. 4). These protocols were believed to be useful in the therapeutic approach to high-risk patients, but both protocols are based on empirical experiences rather than cumulated data which raises doubt on the reliability of each numerical criterion that they have suggested.
Previous studies identified several risk factors for persistent atrial arrhythmias, such as pulmonary hypertension, valvular dysfunction with atrial enlargement, presence of atrial arrhythmia before closure of ASD, and not surprisingly, all these factors are commonly linked to the most important risk factor, the age of the patient.24-26) Therefore, it is very important to close a hemodynamically significant defect early before cascade of deleterious events begin.
In elderly ASD patients with permanent atrial fibrillation, there are 2 therapeutic options which include rhythm control and rate control. Rhythm control involves surgical correction of ASD with Maze procedure at the time of operation, or device closure with catheter ablation. Rate control is achieved by medical treatment including anticoagulation and occasional antiarrhythmic drugs according to the baseline heart rate or arrhythmic event after closure of the defect. A rhythm control policy may be the preferable option in patients with symptomatic tachyarrhythmia.
A recent study on elderly patients with ASD and chronic atrial fibrillation, reported that the outcome of device closure was as good as the outcome from patients without atrial fibrillation in terms of safety, improvement of functional status and reverse remodeling of the heart.27) Their strategy was based on the previous study comparing rhythm control and rate control, which revealed that there was no difference in mortality and quality of life between these strategies (AFFIRM study).28) However, this subject may be controversial since for the time being, the predominant opinion is that rhythm control appears to be superior to rate control in the treatment of atrial fibrillation.29)
For small children, a more sophisticated technical approach and decision making is needed, keeping in mind that the closure system is not specifically designed for the small heart and vessels of very young children. To promote the applicability of device closure, further modification of the device towards decreased rim to waist diameter ratio is warranted.
For elderly patients, thorough evaluation of co-morbidities and risk factors is important to predict post-procedural complication. The physician must let their patients know about the serious complications such as chronic atrial arrhythmia and PAH which may persist after ASD closure so that they can make the right decision in choosing the treatment modality as well as promote compliance to further medical treatment. Also, even though LV dysfunction (LV restriction) after device closure is not frequent, special care must be taken in high risk patients because potentially serious outcomes may be prevented by fastidious evaluation and appropriate treatment plan.
In conclusion, transcatheter closure of secundum ASD with the ASO is technically feasible, safe and effective for many age groups. A meticulous approach and individualized strategy for each patient are mandatory to maximize the efficacy and safety of this versatile therapy.
Figures and Tables
Fig. 1
Discrepancy in the left atrial disc and waist ratio (a/b). This figure shows that in 32 mm ASO, the (a) and (b) ratio is 1.44, but in a 12 mm ASO, the (a) and (b) ratio is 2.17. Relative large rim of LA disc in smaller devices may encroach to adjacent cardiac structures which prevent safe device closure. In reality, these are entirely different devices, when considering the structure and shape. Therefore, the discrepancy maximizes as the device gets smaller. LA disc is marked. a: left atrial disc, b: waist of device. A: anterior-posterior view of 32 mm ASO demonstrates the relationship of left atrial disc and waist size. B: anterior-posterior view of 12 mm ASO reveals that left atrial disc occupies large area in smaller device. ASO: Amplatzer septal occluder.

Fig. 2
The pathophysiologic mechanisms in elderly patients with atrial septal defect. Longstanding left to right shunt in the atrial level results in progressive right heart dilatation, significant TR and subsequent increase in RA pressure. Left heart may also be influenced by chronic volume underload, increased atrial pressure as well as co-morbid diseases. Lt: left, Rt: right, RAE: right atrial enlargement, RVE: right ventricular enlargement, LV: left ventricle, HTN: hypertension, IHD: ischemic heart disease, CO: cardiac output, PAH: pulmonary arterial hypertension, TR: tricuspid regurgitation, RAP: right atrial pressure, LAP: left atrial pressure, LAE: left atrial enlargement, A Fib: atrial fibrillation, A Flut: atrial flutter.
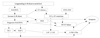
Fig. 3
Different protocols for the detection and management of high risk patients. If left ventricle restriction is detected, both protocols recommend several days of medical therapy including diuretics, inotropic agents and afterload reducing therapy, and if the patient is refractory to medical therapy on a subsequent balloon occlusion test, they recommend using a fenestrated device.5)14)15)23) LAP: left atrial pressure, ASD: atrial septal defect.
References
1. Park MK. Pediatric Cardiology for Practitioners. 2008. 5th ed. Philadelphia: Mosby Elsevier;161–191.
2. Bull C, Deanfield J, de Leval M, Stark J, Taylor JF, Macartney FJ. Correction of isolated secundum atrial septal defect in infancy. Arch Dis Child. 1981. 56:784–786.
3. Lammers A, Hager A, Eicken A, Lange R, Hauser M, Hess J. Need for closure of secundum atrial septal defect in infancy. J Thorac Cardiovasc Surg. 2005. 129:1353–1357.
4. Hijazi ZM, Celiker A. Closure of atrial septal defects. Anadolu Kardiyol Derg. 2005. 5:331.
5. Holzer R, Hijazi ZM. Interventional approach to congenital heart disease. Curr Opin Cardiol. 2004. 19:84–90.
6. Keane JF, Lock JE, Fyler DC. Nadas' Pediatric Cardiology. 2006. 2nd ed. Philadelphia: Saunders Elsevier;603–616.
7. McMahon CJ, Feltes TF, Fraley JK, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter clo-sure. Heart. 2002. 87:256–259.
8. Vogel M, Berger F, Dähnert I, Ewert P, Lange PE. Treatment of atrial septal defects in symptomatic children aged less than 2 years of age us-ing the Amplatzer septal occluder. Cardiol Young. 2000. 10:534–537.
9. Diab KA, Cao QL, Bacha EA, Hijazi ZM. Device closure of atrial septal defects with the Amplatzer septal occluder: safety and outcome in infants. J Thorac Cardiovasc Surg. 2007. 134:960–966.
10. Cardenas L, Panzer J, Boshoff D, Malekzadeh Milani S, Ovaert C. Transcatheter closure of secundum atrial defect in small children. Catheter Cardiovasc Interv. 2007. 69:447–452.
11. Choi JY, Kim NK, Park SJ, Park HK, Park YH, Sul JH. Feasibility and safety of Transcatheter closure of atrial septal defect in small child-ren weighing 10kg or less. Catheter Cardiovasc Interv. 2008. 1:S10. Abstract.
12. Wood AM, Holzer RJ, Texter KM, et al. Transcatheter elimination of left-to-right shunts in infants with bronchopulmonary dysplasia is feasible and safe. Congenit Heart Dis. 2011. 6:330–337.
13. Amin Z. Transcatheter closure of secundum atrial septal defects. Catheter Cardiovasc Interv. 2006. 68:778–787.
14. Spies C, Hijazi ZM. Transcatheter Closure of Secundum Atrial Septal Defects in the Elderly. Korean Circ J. 2009. 39:47–51.
15. Schubert S, Peters B, Abdul-Khaliq H, Nagdyman N, Lange PE, Ewert P. Left ventricular conditioning in the elderly patient to prevent congestive heart failure after transcatheter closure of atrial septal defect. Catheter Cardiovasc Interv. 2005. 64:333–337.
16. Swan L, Varma C, Yip J, et al. Transcatheter device closure of atrial septal defects in the elderly: technical considerations and short-term outcomes. Int J Cardiol. 2006. 107:207–210.
17. Elshershari H, Cao QL, Hijazi ZM. Transcatheter device closure of atrial septal defects in patients older than 60 years of age: immediate and follow-up results. J Invasive Cardiol. 2008. 20:173–176.
18. Shah D, Azhar M, Oakley CM, Cleland JG, Nihoyannopoulos P. Na-tural history of secundum atrial septal defect in adults after medical or surgical treatment: a historical prospective study. Br Heart J. 1994. 71:224–227.
19. Attie F, Rosas M, Granados N, Zabal C, Buendía A, Calderón J. Surgical treatment for secundum atrial septal defects in patients >40 years old: a randomized clinical trial. J Am Coll Cardiol. 2001. 38:2035–2042.
20. Miyaji K, Furuse A, Tanaka O, Kubota H, Ono M, Kawauchi M. Surgical repair for atrial septal defect in patients over 70 years of age. Jpn Heart J. 1997. 38:677–684.
21. Shibata Y, Abe T, Kuribayashi R, et al. Surgical treatment of isolated secundum atrial septal defect in patients more than 50 years old. Ann Thorac Surg. 1996. 62:1096–1099.
22. Yalonetsky S, Lorber A. Comparative changes of pulmonary artery pressure values and tricuspid valve regurgitation following transcatheter atrial septal defect closure in adults and the elderly. Congenit Heart Dis. 2009. 4:17–20.
23. Ewert P, Berger F, Nagdyman N, et al. Masked left ventricular restriction in elderly patients with atrial septal defects: a contraindication for closure. Catheter Cardiovasc Interv. 2001. 52:177–180.
24. Gatzoulis MA, Freeman MA, Siu SC, Webb GD, Harris L. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999. 340:839–846.
25. Oliver JM, Gallego P, Gonzlez A, Benito F, Mesa JM, Sobrino JA. Predisposing conditions for atrial fibrillation in atrial septal defect with and without operative closure. Am J Cardiol. 2002. 89:39–43.
26. Silversides CK, Siu SC, McLaughlin PR, et al. Symptomatic atrial arrhythmias and transcatheter closure of atrial septal defects in adult patients. Heart. 2004. 90:1194–1198.
27. Taniguchi M, Akagi T, Ohtsuki S, et al. Transcatheter closure of atrial septal defect in elderly patients with permanent atrial fibrillation. Catheter Cardiovasc Interv. 2009. 73:682–686.
28. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002. 347:1825–1833.
29. Verma A, Natale A. Should atrial fibrillation ablation be considered first-line therapy for some patients? Why atrial fibrillation ablation should be considered first-line therapy for some patients. Circulation. 2005. 112:1214–1222.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download