Abstract
Stress-induced cardiomyopathy is a unique reversible cardiovascular disease precipitated by acute emotional or physical stress. It is associated with a high prevalence of chronic anxiety disorder that precedes the onset of cardiomyopathy, as well as comorbid cardiovascular risk factors that are similar to the ST segment elevation of myocardial infarction. A thirty-five-year-old woman suffering from anorexia nervosa visited our hospital complaining of severe general weakness. She was diagnosed with stress-induced cardiomyopathy and mural thrombus using a transthoracic echocardiogram. Therefore, she was given anticoagulation therapy and nutrition with immediate psychiatric interventions. After two weeks of treatment, the follow-up echocardiogram indicated a significant improvement of the left ventricular dysfunction and mural thrombus.
Anorexia nervosa (AN) is characterized by a decrease in caloric intake, weight loss, amenorrhea and behavioral changes. According to Morris and Twaddle,1) eighty percent of AN patients have cardiovascular complications, such as bradycardia, hypotension, arrhythmia and repolarization abnormalities, and ten percent of them experience sudden cardiac death. Stress-induced cardiomyopathy (SICMP) is designated after echocardiographic abnormalities that are characterized by extensive akinesia (ballooning) of the apical region and hypercontraction of the basal segment of the left ventricle (LV).2) Patients with SICMP demonstrate cardiac enzyme abnormalities and electrocardiographic (ECG) changes similar to those seen in acute myocardial infarction (MI) without significant luminal narrowing of the coronary arteries on the coronary angiography.3) SICMP is a very rare complication of AN. We report a rare case of SICMP caused by separation anxiety as a precipitating event in AN.
A 35-year-old woman came to our emergency department complaining of severe general weakness and epigastric pain for the last 5 days. In her past medical history, she was diagnosed with AN and borderline personality disorder at a local psychiatric clinic 5 years ago. The only meals she used to have on a daily basis were milk and fruits for a period of 17 years, and even her daily meal intake rapidly decreased after her mother went abroad two weeks ago. This was because she appeared to be extremely dependent on her mother. She did not have any history of diabetes mellitus, hypertension, or hepatitis previously. However, she had taken psychiatric medications for 6 months approximately 5 years ago. In addition, she also intermittently took medications for amenorrhea and osteoporosis.
On admission, her vital signs were: blood pressure of 90/60 mm Hg, pulse rate of 60 beats/min, respiration rate of 20 breaths/min, and body temperature of 36.5℃. She was alert, but looked chronically ill and cachexic. Her body weight was 30 kg and body mass index was 11.71 kg/m2. She had a severely dehydrated tongue, but her chest and abdomen physical examination showed no abnormalities. Her laboratory examinations were as follows; white blood cell 8,940/mm3, hemoglobin 15.8 g/dL, hematocrit 43.8%, platelet 70,000/mm3, random blood glucose 132 mg/dL, blood urea nitrogen/creatinine 35.9/0.44 mg/dL, total protein/albumin 5.6/3.6 g/dL, aspartate aminotransferase/alanine aminotransferase 471/374 IU/L, total bilirubin 1.90 mg/dL, Na/K 127/2.6 mEq/L, Ca/P/Mg 8.5/5.3/1.6 mg/dL, total cholesterol 116 mg/dL, triglyceride 38 mg/dL, high density lipoprotein-cholesterol 74 mg/dL, low density lipoprotein-cholesterol <10 mg/dL, T3 0.43 ng/mL (0.78-1.82 ng/mL), free T4 0.94 ng/mL (0.85-1.86 ng/mL), thyroid stimulating hormone 1.71 mIU/L (0.17-4.05 mIU/L), adrenocoticotropic hormone 11.44 pg/mL (6.00-56.70 pg/mL), cortisol 17.40 ug/dL (9.41-26.06 ug/dL), and aldosterone 31.66 pg/mL (40-102 pg/mL). Initial cardiac biomarkers were elevated as follows; creatine kinase-MB 154.30 ng/mL (0-5 ng/mL), troponin-I 2.580 ng/mL (0-0.78 ng/mL) and N-terminal pro-B-type natriuretic peptide 8,963 pg/mL (0-155 pg/mL). Her chest X-ray and abdomen computed tomography showed no significant lesions associated with epigastic pain. Initial electrocardiogram showed sinus rhythm (60 beats/min), prolonged corrected QT interval (543 ms), pathologic Q waves in II, III, aVF and V1-V3, inverted T waves in II, III and aVF and poor R progression in precordial leads (Fig. 1A). Echocardiogram revealed large akinetic areas around the apical, inferior, anterior and lateral sides with hypercontraction of the basal segments, linear echogenic mural thrombus, pericardial effusion, and a reduction of ejection fraction to 36% (Fig. 2). Therefore, we strongly suspected that she was SICMP because of her characteristic echocardiograhic findings.
Only standard medical treatment was given to the patient since she and her guardian refused to receive a coronary angiogram. Therefore, we started the treatment with low molecular weight heparin, and nutrition support with immediate multi-systemic interventions. Psychiatric consultation was also accompanied by medical therapy. After three days, we stopped anticoagulation therapy due to aggravated thrombocytopenia and prolonged prothrombin time (PT). Overall, her daily oral intake increased together with her body weight, 33 kg compared to 30 kg in the beginning. Electrolyte imbalance, liver dysfunction, and thrombocytopenia were also normalized. Two weeks later, the next follow-up echocardiogram indicated a significant improvement of the LV ejection fraction (42%) and wall motion abnormalities. On the other hand, apical mural thrombosis still remained and pericardial effusion increased (Fig. 3). On the next follow-up electrocardiogram, the corrected QT interval decreased to 453 ms. Furthermore, pathologic Q waves, inverted T waves and poor R progression observed in the initial ECG disappeared (Fig. 1B). However, she and her guardian strongly insisted on discharging from the hospital against the clinician's recommendation. Although appointments with cardiology and psychiatry departments were arranged with continuing mental support and follow-up echocardiogram, she never came back to the hospital after her discharge.
AN is characterized by a decrease in caloric intake, weight loss, amenorrhea and behavioral changes. Most AN patients are adolescents with a mean age of 15 years old.1) Patients with AN may also suffer from bone marrow suppression, liver failure and numerous metabolic complications, and it is assumed that these complications are caused by severe malnutrition.4) Eighty percent of these patients have cardiovascular complications, such as bradycardia, hypotension, arrhythmia and repolarization abnormalities.5) Furthermore, 10% of them experience sudden cardiac death.6) Myocardial mass decreases together with body weight, and results in a reduction in LV ejection fraction and diffuse global wall motion abnormalities. Myocardial damage is reversible with a tolerable prognosis since body weight can be regained.7)
SICMP is characterized by a transient mid ventricular dysfunction and apical akinesis, diskinesis with the basal function preserved, or hyperkinetic. The latter is a rare case of AN only found in a few case reports.8)9) Some patients with SICMP are complicated with mural thrombosis as seen in acute MI. According to Haghi et al.10) 8% of SICMP patients had mural thrombosis in the LV. However, patients who have SICMP with mural thrombosis have not ever been reported in Korea. In our study, low molecular weight heparin was infused after admission, and on the third day, anticoagulant therapy had to be halted because of no improvement of thrombocytopenia and PT prolongation. Only intravenous nutritional support and correction of electrolyte imbalance were sustained.
Recurrence rate of SICMP with a precipitant as psychiatric disorder is supposed to be higher than that of previously published data,11-13) because physical or emotional stress related to the psychiatric disorder is not transient. Treatment of SICMP especially in AN patients who also suffer from other complications after severe malnutrition should be investigated with multi-systemic approaches.
Figures and Tables
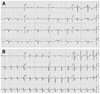 | Fig. 1Electrocardiogram on admission and follow-up. A: the electrocardiogram revealed significant QTc prolongation and T wave inversion in leads II, III, aVF and V1-V6 on admission day. It also showed pathologic Q wave in leads II, III, aVF and V1-V3. B: follow-up electrocardiogram revealed normal sinus rhythm with non-specific ST segment change on the 7th hospital day. |
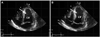 | Fig. 2Transthoracic echocardiogram on admission. A: the photograph demonstrates a large akinetic area around the apex during diastole with linear echogenic mural thrombus (arrow) surrounding septal apex. B: this photograph showed hypercontraction of the basal segments with reduction of ejection fraction to 36% during systole. |
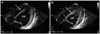 | Fig. 3Follow-up transthoracic echocardiogram. A: the previous akinetic left ventricular walls showed an improvement on the wall motion during diastole, and the mural thrombus decreased (arrow). B: during systole, the improvement of the systolic left ventricular function was noticed: ejection fraction increased to 59% and pericardial effusion increased. |
References
1. Morris J, Twaddle S. Anorexia nervosa. BMJ. 2007. 334:894–898.
2. Satoh H, Tateishi H, Uchida T. Kodama K, Haze K, Hon M, editors. Takotshubo-type cardiomyopathy due to multivessel spasm. Clinical Aspect of Myocardial Injury: from Ischemia to Heart Failure. 1990. Tokyo: Kagakuhyouronsya;56–64.
3. Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol. 2001. 38:11–18.
4. Saito S, Kita K, Morioka CY, Watanabe A. Rapid recovery from anorexia nervosa after a life-threatening episode with severe thrombocytopenia: report of three cases. Int J Eat Disord. 1999. 25:113–118.
5. Isner JM, Roberts WC, Heymsfield SB, Yager J. Anorexia nervosa and sudden death. Ann Intern Med. 1985. 102:49–52.
6. Cooke RA, Chambers JB. Anorexia nervosa and the heart. Br J Hosp Med. 1995. 54:313–317.
7. Mont L, Castro J, Herreros B, et al. Reversibility of cardiac abnorma-lities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry. 2003. 42:808–813.
8. Ohwada R, Hotta M, Kimura H, et al. Ampulla cardiomyopathy after hypoglycemia in three young female patients with anorexia nervosa. Intern Med. 2005. 44:228–233.
9. Rotondi F, Manganelli F, Lanzillo T, et al. Tako-tsubo cardiomyopathy complicated by recurrent torsade de pointes in a patient with anorexia nervosa. Intern Med. 2010. 49:1133–1137.
10. Haghi D, Papavassiliu T, Heggemann F, Kaden JJ, Corggrefe M, Suselbeck T. Incidence and clinical significance of left ventricular thrombus in tako-tsubo cardiomyopathy assessed with echocardiography. QJM. 2008. 101:381–386.
11. Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007. 50:448–452.
12. Hong KS. Stress-induced cardiomyopathy: a need for rospective multicenter trials. Korean Circ J. 2010. 40:258–259.
13. Lee HH, Gwon HC, Kim BJ, et al. Clinical manifestation of novel stress-induced cardiomyopathy mimicking acute myocardial infarction. Single center prospective registry. Korean Circ J. 2002. 32:1054–1063.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download