Abstract
Background and Objectives
In our previous study, we found that the gene transfer of a potent derivative of cartilage oligomeric matrix protein Angiopoietin-1 (COMP-Ang-1) substantially prevented hypertension, microvascular rarefaction, and target organ damage in spontaneously hypertensive rats (SHRs). The purpose of the present study was to examine the role of nitric oxide (NO) in the therapeutic effects observed after COMP-Ang-1 gene transfer.
Materials and Methods
To exclude the NO-mediated effects in COMP-Ang-1 gene therapy, the SHRs were treated with an NO synthase (NOS) inhibitor, Nw-nitro-L-arginine methyl ester (L-NAME) before the electrophoretic gene transfer.
Results
The pretreatment with L-NAME induced a severe and sustained increase in systolic blood pressure (BP) in a LacZ plasmid transferred control SHR. However, the electrophoretic transfer of a COMP-Ang-1 plasmid instead of LacZ plasmid in L-NAME-pretreated SHRs substantially blocked the development of hypertension without any significant difference in comparison with L-NAME-untreated COMP-Ang-1 plasmid transferred groups. In addition, the COMP-Ang-1 plasmid transfer substantially attenuated microvascular rarefaction and arteriole remodeling in the heart and kidney, which might account for the mild histological alterations observed in the COMP-Ang-1 plasmid transferred group, in contrast to the severe fibrosis and necrosis seen in the LacZ plasmid controls.
Conclusion
These therapeutic outcomes of COMP-Ang-1 gene transfer even in NOS inhibited SHRs suggested that the antihypertensive effect of COMP-Ang-1 was not merely secondary to NO-mediated vasorelaxation, but it may be associated with its ability to protect the vascular endothelium probably via an NO-independent mechanism which serves to attenuate microvascular rarefaction and target organ damage, and also to prevent hypertension by reducing peripheral vascular resistance.
Hypertension is a cardiovascular risk factor in which sustained high blood pressure (BP) causes constriction of the microvasculature, making it unperfused and thereby, leading to the dysfunction of target organs such as the heart and kidney.1)2) In particular, these hypertension-related structural and functional alterations of the microvasculature termed as microvascular rarefaction, also increase peripheral vascular resistance, which further elevate the BP.2)3) To break the vicious cycle between hypertension and microvascular rarefaction, many therapeutic approaches have been focused on lowering BP using adrenergic receptor antagonists, calcium channel blockers and others.4) However, few studies have focused on breaking the cycle by protecting the vascular endothelium against a variety of stress factors in order to inhibit microvascular rarefaction and the subsequent development of hypertension.5)
With this in mind, we previously investigated the therapeutic effect of an endothelial survival factor, Angiopoietin-1 (Ang-1), in prevention of hypertension and target organ da-mage in spontaneously hypertensive rats (SHRs), a model of essential hypertension characterized by vascular rarefaction and target organ damage.6)
In that particular study, the electroporation-mediated transfer of a plasmid encoding a strong and potent variant of Ang-1, cartilage oligomeric matrix protein (COMP)-Ang-1, was found to be effective in preventing hypertension and reducing target organ damage in SHRs. Of note, COMP-Ang-1 was shown to substantially increase the plasma level of nitrite, a metabolite of nitric oxide (NO), through the endothelial-specific Tie2/endothelial NO synthase (eNOS) signaling pathway.
NO plays an important role in controlling BP by regulating vasodilation and it is also important for maintaining endothelial homeostasis. Therefore, the increased NO synthesis by COMP-Ang-1 was postulated to be a possible underlying mechanism for the beneficial effects of COMP-Ang-1, although further experiments were needed to support this interpretation.
The present study aimed to investigate whether or not NO was a key player in the overall therapeutic effects of COMP-Ang-1 in SHRs. To address this question, SHRs were pretreated with an NOS inhibitor, Nw-nitro-L-arginine methyl ester (L-NAME), in order to exclude the NO-mediated effects of COMP-Ang-1. In NOS inhibited SHRs, BP and histopathological changes in target organs were evaluated following COMP-Ang-1 plasmid transfer.
Six-week-old male SHRs (Charles River Laboratories, Yokohama, Japan) were randomized for how many electrophoretic transfers (eight electric pulses of 200 V/cm for 50 ms at 1 Hz using an ECM 830 electroporator; BTX Division of Genetronics, San Diego, CA, USA) of plasmids encoding LacZ (pLacZ, the control plasmid) or COMP-Ang-1plasmid (pCO-MP-Ang-1) (100 µg of plasmid in 100 mL of half-saline solution) into the adductor muscle, as previously described.6) For chronic NOS inhibition, SHRs were restricted to drinking water containing 50 mg/L of L-NAME (Sigma, St. Louis, MO, USA) from one week before the gene transfer until the day of sacrifice.7)8)
For surgical procedures, rats were anesthetized with an intraperitoneal injection of ketamine-xylazine (50 mg/kg and 2 mg/kg, respectively). Systolic BP was measured non-invasively how frequently using the tail-cuff method (IITC Life Science Instruments, Woodland Hills, CA, USA) in conscious rats on three different occasions. All rats were acclimated to the system one week before starting the experiment. The animal experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published in the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
The concentration of nitrite, a metabolite of NO, in the blood was measured with an NO assay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. As NO is rapidly metabolized mainly to nitrite or nitrate, the concentrations of both these anions were used as a quantitative measure of NO production. Briefly, nitrate was converted into nitrite by nitrate reductase, and then the amount of nitrite was measured spectrophotometrically after adding Griess reagent.
The hearts and kidneys were fixed in 4% paraformaldehyde and embedded in paraffin, or frozen in cryofreezing medium. After staining with Masson's Trichrome stain (MT), the histological changes (e.g., fibrosis, necrosis, and vascular occlusion) in the ventricular walls of the hearts and the cortices of the kidneys were examined. For the evaluation of capillary density, the heart and kidney sections were stained with mouse anti-rat CD31 antibody (BD Pharmingen, San Diego, CA, USA) and mouse anti-rat RECA-1 antibody (Abcam, Cambridge, UK), respectively. After incubating sections with biotinylated anti-mouse antibody (Jackson ImmunoResearch, West Grove, PA, USA), positive immunoreactivity was visualized using ABC-peroxidase kits (ChemMate™ DAKO Envision™ Detection kit, DAKO, Copenhagen, Denmark). Controls were prepared using class- and species-matched nonspecific antibodies.
Urine was collected weekly from each rat housed in a metabolic cage for 24 hours. Protein levels in urine were measured using a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA).
All data are presented as mean±standard error of mean (SEM). One-way analysis of variance was used to determine the significance of differences between groups; where appropriate, data were analyzed using post hoc Student's t-tests for unpaired observations and the Bonferroni correction for multiple comparisons. p<0.05 was accepted as significant. The number of samples examined in each experiment is indicated by 'n'.
To investigate the antihypertensive effect of COMP-Ang-1 in NOS inhibited SHRs, plasmids encoding COMP-Ang-1 or LacZ were electrophoretically transferred into L-NAME-pretreated SHRs, and the secretion of expressed COMP-Ang-1 protein into the bloodstream was confirmed by as was done in our previous study (data not shown). In agreement with other reports on L-NAME-pretreated SHRs, Fig. 1A shows that chronic NOS inhibition induced a sustained 10-15 mm Hg increase in the systolic BP of pLacZ-transferred SHRs compared to L-NAME-pretreated pLacZ SHRs (162.3±3.7 mm Hg in pLacZ transferred SHRs, 175.9±6.0 mm Hg in L-NAME-pretreated pLacZ SHR).7)8) However, the electrophoretic transfer of pCOMP-Ang-1 substantially blocked the development of hypertension in NOS inhibited SHRs. Of note, the systolic BP of L-NAME-pretreated pCOMP-Ang-1-transferred SHRs was not significantly different from that of L-NAME-untreated pCOMP-Ang-1-transferred SHRs (p=0.0876, n=11), which implies that COMP-Ang-1 gene transfer prevented the elevation of BP mainly in an NO-independent manner. In other words, the antihypertensive effects of COMP-Ang-1 might not occur through NO-mediated vasorelaxation. The introduction of L-NAME into the SHRs completely abolished the effect of COMP-Ang-1 on NO production, as shown in Fig. 1B; there was no difference in the plasma levels of nitrite between pLacZ- and pCOMP-Ang-1-transferred SHRs.
To analyze the effect of COMP-Ang-1 on microvascular rarefaction in NOS inhibited SHRs, the vasculature in target organs such as the heart and kidney was evaluated by immunohistochemistry using endothelial specific CD31 or RECA antibody. As shown in Fig. 2A and B, the capillary number was significantly higher in the hearts from pCOMP-Ang-1-transferred SHRs than in the hearts from pLacZ-transferred controls. Similar results were seen in the kidneys as well: pCOMP-Ang-1-transferred SHRs had more peritubular capillaries and better preserved glomeruli with an intact endothelium than pLacZ-transferred control (Fig. 2C). This difference in the microvascular rarefaction between pCOMP-Ang-1-and pLacZ-transferred SHRs may reflect in the histopathological differences in their target organs, as described below.
When target organ damage was examined in NOS inhibited SHRs (Fig. 3), the hearts of L-NAME pretreated pLacZ-transferred SHRs were found to have markedly granulated tissues and multifocal areas of myocardial fibrosis with obliterative arteriosclerosis in small arterioles. In contrast, the hearts of L-NAME pretreated pCOMP-Ang-1-transferred SHRs showed only mild alterations in the microvasculature and fewer instances of myocardial fibrosis (Fig. 3A and B). In the kidney, L-NAME pretreated pLacZ-transferred SHRs exhibited more glomerular fibrinoid necrosis and tubular interstitial fibrosis than L-NAME-pretreated pCOMP-Ang-1-transferred SHRs, which may explain the high protein content in their urine (Fig. 3C and D). In addition, the renal vasculature in L-NAME-pretreated pLacZ SHRs showed characteristics similar to those found in the heart, such as a near-complete occlusion of the lumen in small arterioles rather than in large renal arteries. As these precapillary arterioles are recognized as the critical determinants of peripheral vascular resistance through their effects on hydrostatic pressure, their wide vascular lumen and high number in L-NAME-pretreated pCOMP-Ang-1 SHRs seem to contribute to preventing the development of hypertension in L-NAME-pretreated SHRs.
In our previous study, a strong endothelial survival factor, COMP-Ang-1, was found to ameliorate microvascular rarefaction and tissue damage in the heart and kidney, and to prevent the development of hypertension in a genetic hypertension animal model.6) In particular, the high levels of NO in pCOMP-Ang-1-transferred SHRs have led us to postulate that COMP-Ang-1 lowered the systolic BP by enhancing NO-mediated vascular relaxation, which may result in the attenuation of microvascular rarefaction and target organ damage. However, the present results obtained in L-NAME-pretreated SHRs showed that COMP-Ang-1 still significantly prevented the elevation of BP in SHRs when NO synthesis was inhibited. This suggested that the antihypertensive effects of COMP-Ang-1 were not merely secondary to NO-mediated vasorelaxation, but they may be associated with attenuated microvascular rarefaction.
Although endothelium-derived NO plays a major role in controlling arterial BP through endothelium-dependent vasorelaxation, microvascular rarefaction is another important factor determining BP by increasing peripheral vascular resistance.2) Since peripheral vascular resistance is mainly regulated at the distal vasculature such as small arterioles and capillaries where a substantial drop in intravascular pressure occurs, a thorough examination of the microvasculature in L-NAME-pretreated SHRs was performed. It was found that pCOMP-Ang-1-transferred SHRs displayed normal-looking arterioles and had a high capillary density, while pLacZ-transferred SHRs exhibited 'onion skin' like arteriosclerotic occlusion and severe capillary rarefaction frequently. This difference in the microvascular structure may directly contribute to preventing BP elevation in pCOMP-Ang-1-transferred SHRs. Apart from determining peripheral vascular resistance, the microvasculature functions to provide sufficient nutrients and oxygen to the surrounding tissues in response to tissue demand.3) In this regard, attenuated microvascular rarefaction in pCOMP-Ang-1-transferred SHRs may account for the observed significant preservation of the histological structure and function in the heart and kidney
Although the eNOS/NO pathway plays a crucial role in maintaining endothelial homeostasis as well as in regulating vascular tone, several studies have reported that hypertension and arterial rarefaction in eNOS knockout mice were completely rescued by hyralazine hydralazine, a non-specific vasodilator.9)10) These data indicated that NO plays a major role in hypertension by regulating systemic BP through enhancing vasodilation, but that this role does not include the maintenance of microvascular structure by protecting the endothelium from other hypertensive stresses. Considering this role of NO in hypertension, the antihypertensive effects induced by COMP-Ang-1 gene transfer in NOS inhibited SHRs would be secondary to the endothelial protective effects induced by pCOMP-Ang-1 transfer, and this should lead to a better preserved microvasculature.
In a variety of animal models, COMP-Ang-1 has been shown to enhance endothelial survival under vascular stress. COMP-Ang1 protein treatment has been reported to ameliorate renal injury and fibrosis by protecting peritubular capillaries and inhibiting inflammation.11) COMP-Ang-1 has been also shown to protect the capillary endothelium of the intestinal villi from radiation-induced apoptosis.12) In particular, COMP-Ang1 was found to significantly activate phosphatidylinositol 3' kinase/Akt pathway; one of the well-known signaling pathways promoting endothelial survival and inhibiting endothelial apoptosis. In this regard, such a role of COMP-Ang1 in endothelial protection seems to be associated with Akt signaling pathway. However, COMP-Ang-1 might not protect the endothelium via eNOS/NO pathway, since COMP-Ang-1 enhances angiogenesis and blood flow even in eNOS or inducible NOS knockout mice. This result indicates that the activation of the eNOS/NO cascade might not be required for its actions on the endothelium.13)
Taken together, our results suggest that the antihypertensive effects of pCOMP-Ang-1 gene transfer in SHRs might arise from the preservation of microvasculature, which in turn would be expected to attenuate target organ damage as well. Recently, microvascular abnormalities in hypertensive patients have been appreciated as a cause, and not merely a consequence of hypertension. Indeed, several recent studies have reported that treatment of SHRs with a potent vasodilator, hydralazine, significantly decreased the BP, but did not ameliorate cardiac remodeling and renal failure, suggesting that controlling BP itself does not fully prevent or cure hypertension-related target organ damage.14)15) In this regard, endothelial survival factors aimed at protecting the microvasculature could therefore serve as new antihypertensive agents with therapeutic advantages in preventing hypertension-associated target organ damage above and beyond those of the treatment aimed at controlling BP directly.16-19)
Figures and Tables
Fig. 1
pCOMP-Ang-1 gene transfer ameliorates hypertension in NOS inhibited SHRs. A: a significant hypotensive effect of pCOMP-Ang-1 gene transfer was seen in L-NAME-pretreated SHRs. No significant difference (p=0.0876, n=11) was observed between L-NAME-pretreated pCOMP-Ang-1-transferred SHRs (pCOMP-Ang-1+L-NAME, 161.8±2.4 mm Hg) and L-NAME untreated pCOMP-Ang-1-transferred SHRs (pCOMP-Ang-1, 154.2±2.9 mm Hg). However, systolic BP of pCOMP-Ang-1+L-NAME SHRs was significantly (n=11) lower than that of L-NAME pretreated pLacZ-transferred SHRs (pLacZ+L-NAME, 162.3±3.7 mm Hg). The systolic BP of SHRs (in mm Hg) was measured using the tail-cuff method just before (white bar) and three weeks after (black bar) gene transfer. B: no significant difference (NS) was seen in the plasma concentration of nitrites between pLacZ+L-NAME (12.2±0.6 mM) and pCOMP-Ang-1+L-NAME SHRs (12.1±1.0 mM) (n=11).

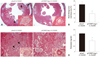
Fig. 2
pCOMP-Ang-1 gene transfer reduces microvascular rarefaction in NOS inhibited SHRs. The microvasculature of the heart (A: red arrowheads indicating the absence of blood vessels) and kidney (C: red arrowheads indicating the loss of glomerular capillaries) was visualized by immunohistochemical staining with CD31 antibody for frozen sections or RECA antibody for paraffin sections. B: the capillary density in the heart section was quantified per myofiber (1.3±0.04 cells/myofiber in pCOMP-Ang-1+L-NAME, 0.8±0.09 cells/myofiber in pLacZ+L-NAME, *p<0.05, n=7). Scale bar is 10 µm.
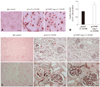
Fig. 3
pCOMP-Ang-1 gene transfer attenuates myocardial and renal damage in NOS inhibited SHRs. Representative photomicrographs of the MT-stained heart (A: black arrowheads indicating the multifocal myocardial fibrosis) and kidney (C: black arrowheads indicating the tubular interstitial fibrosis) with high-power images of small arterioles (right bottom insets). B: the fibrotic area shown in the MT-stained heart sections was quantified as a percentage relative to the total tissue area (20.7±3.0% in pCOMP-Ang-1+L-NAME, 42.2±0.08% in pLacZ+L-NAME, *p<0.05, n=7). D: Kidney function was determined by measuring the amount of protein in the urine collected at the end of the experiment (1.0±0.1 mg/mL in pCOMP-Ang-1+L-NAME, 1.6±0.03 mg/mL in pLacZ+L-NAME, *p<0.05, n=4). Scale bar is 10 µm.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0017233) and by a grant of the Korea Healthcare Technology R & D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084072). All authors confirm that there is no conflict of interest associated with this publication.
References
1. Cohuet G, Struijker-Boudier H. Mechanisms of target organ damage caused by hypertension: therapeutic potential. Pharmacol Ther. 2006. 111:81–98.
2. Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001. 104:735–740.
3. Mourad JJ, Laville M. Is hypertension a tissue perfusion disorder? Implications for renal and myocardial perfusion. J Hypertens Suppl. 2006. 24:S10–S16.
4. Battegay EJ, de Miguel LS, Petrimpol M, Humar R. Effects of anti-hypertensive drugs on vessel rarefaction. Curr Opin Pharmacol. 2007. 7:151–157.
5. Redon J. Antihypertensive treatment: should it be titrated to blood pressure reduction or to target organ damage regression? Curr Opin Nephrol Hypertens. 2005. 14:448–452.
6. Lee JS, Song SH, Kim JM, et al. Angiopoietin-1 prevents hypertension and target organ damage through its interaction with endothelial Tie2 receptor. Cardiovasc Res. 2008. 78:572–580.
7. Olzinski AR, McCafferty TA, Zhao SQ, et al. Hypertensive target organ damage is attenuated by a p38 MAPK inhibitor: role of systemic blood pressure and endothelial protection. Cardiovasc Res. 2005. 66:170–178.
8. Nakamura Y, Ono H, Zhou X, Frohlich ED. Angiotensin type 1 receptor antagonism and ACE inhibition produce similar renoprotection in N(omega)-nitro-L>-arginine methyl ester/spontaneously hypertensive rats. Hypertension. 2001. 37:1262–1267.
9. Kubis N, Besnard S, Silvestre JS, et al. Decreased arteriolar density in endothelial nitric oxide synthase knockout mice is due to hypertension, not to the constitutive defect in endothelial nitric oxide synthase enzyme. J Hypertens. 2002. 20:273–280.
10. Kubis N, Richer C, Domergue V, Giudicelli JF, Lévy BI. Role of microvascular rarefaction in the increased arterial pressure in mice lacking for the endothelial nitric oxide synthase gene (eNOS3pt-/-). J Hypertens. 2002. 20:1581–1587.
11. Kim W, Moon SO, Lee SY, et al. COMP-angiopoietin-1 ameliorates renal fibrosis in a unilateral ureteral obstruction model. J Am Soc Nephrol. 2006. 17:2474–2483.
12. Cho CH, Kammerer RA, Lee HJ, et al. Designed angiopoietin-1 variant, COMP-Ang1, protects against radiation-induced endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004. 101:5553–5558.
13. Cho CH, Sung HK, Kim KT, et al. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci U S A. 2006. 103:4946–4951.
14. Kobori H, Ozawa Y, Suzaki YY, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005. 16:2073–2080.
15. Du WM, Miao CY, Liu JG, Shen FM, Yang XQ, Su DF. Effects of long-term treatment with ketanserin on blood pressure variability and end-organ damage in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2003. 41:233–239.
16. Noon JP, Walker BR, Webb DJ, et al. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997. 99:1873–1879.
17. Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999. 34:655–658.
18. Antonios TF, Rattray FM, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart. 2003. 89:175–178.
19. Jung AD, Kim W, Park SH, et al. The effect of telmisartan on endothelial function and arterial stiffness in patients with essential hypertension. Korean Circ J. 2009. 39:180–184.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download