Abstract
Background and Objectives
Dysfunction of arteriovenous fistulas (AVFs) and arteriovenous grafts (AVGs) contributes significantly to morbidity and hospitalization in the dialysis population. We evaluated the primary patency of AVFs following percutaneous transluminal angioplasty (PTA) in haemodialysis patients.
Subjects and Methods
We performed 231 interventions in 118 patients with a mean age of 62.1±12.9 years. We performed 122 interventions in 53 AVG patients (44.9%), and 109 interventions in 65 AVF patients (55.1%). If there was thrombosis of the vascular access, urokinase was administered and/or thrombus aspiration was performed. The stent was inserted when balloon dilatation did not expand sufficiently or elastic recoil occurred.
Results
For the 118 patients, the median patency time was 10.45±10.29 months at 92 months of follow-up. The primary patencies for stenotic AVFs at 6, 12, 24, 36, 48, and 60 months were 63.4%, 41.4%, 17.0%, 9.7%, 7.3%, and 2.4%, respectively. The primary patencies for AVGs at 6, 12, 24, and 36 months were 36.9%, 19.5%, 10.8%, 2.1%, respectively, and were obtained by means of the Kaplan-Meier analysis (log rank=6.42, p<0.05). The median patency time was 11.0 months and 4.45 months in the non-thrombus and thrombus groups, respectively. The complication rate was 1.73% (4/231); two cases of pseudoaneurysms and two cases of extravasation were detected. All therapy failures (5/231) occurred in thrombotic lesions of AVGs and were treated surgically.
Hemodialysis can be performed via central venous catheters (internal jugular, subclavian and femoral vein) or via a permanent arteriovenous access. Long-term patency of central catheters is low, because they are prone to infection and thrombosis.1) The stenosis and subsequent thrombosis of arteriovenous fistulas (AVFs) may lead to a loss of vascular access sites. Dysfunction of AVFs and arteriovenous grafts (AVGs) occurs frequently in haemodialysis patients and contributes significantly to morbidity and hospitalization in the dialysis population.2)
In recent years, percutaneous interventional techniques have been tried and established as a viable alternative means in the management of fistula dysfunction. In recent years, several studies3-9) have demonstrated that angioplasty is efficacious with some advantages compared to the conventional surgical treatment such as a shorter time needed to perform the procedure and shorter hospitalization, less discomfort for the patient, and lower infection rates. Additionally, it enables dialysis immediately after the procedure without the necessity of using a central venous catheter.
In Korea, however, the data regarding the results of percutaneous transluminal angioplasty (PTA) in dysfunctional fistulas is limited. In this study, we evaluated the primary patency of AVFs after PTA in haemodialysis patients.
We performed 231 interventions in 118 patients on hemodialysis for chronic renal failure. There were 65 men and 53 women with a mean age of 62.1±12.9 years (range, 28-86 years). Patients with malfunctioning arteriovenous access who presented at the Combined Internal Medicine/Interventional Radiology Service at Mokdong Hospital, School of Medicine of Ewha Womans University were followed between January 2002 and September 2009. The drop-out rate was 23.7% (28 patients) (Table 1).
A 5 to 7 Fr introducer sheath was inserted anterogradely or retrogradely according to the site of stenosis and angiography was performed. Diagnostic angiography was performed through the sheath. A hydrophilic-coated, steerable, 0.035-inch Terumo guidewire (Terumo Corp., Tokyo, Japan) was passed. A balloon catheter was then passed over the guidewire and advanced to the most central lesion. According to the size of the veins, 4-, 6-, 8-, 10-, and 12-mm balloons were used. Balloon size was determined based on the findings on fistulography or the known graft diameter (Fig. 1). A pressure of 10 atm was routinely utilized. If this was not effective in breaking the lesion, pressures of 15 and 20 atm were sequentially applied. The balloon was allowed to remain inflated for 1-2 minutes with each inflation. Multiple inflations were used for resistant lesions. The choice of the appropriate percutaneous approach depends on the size and location of the thrombus detected by angiography. A short segment thrombosis can be simply treated with balloon angioplasty alone; however, an extensive thrombosis requires the combination of mechanical devices and/or thrombolytic agents with consecutive balloon angioplasty. Delivery of 100,000 to 400,000 U of urokinase directly into the thrombus was performed in our patients with use of the pre-existing catheter. For thrombosed fistulas, a manual catheter-based thromboaspiration and/or mechanical thrombectomy with an Arrow-Trerotola percutaneous thrombolytic device (ARROW International Inc., Reading, PA, USA) was performed (Fig. 2). Despite the complete expansion of the balloon (without a waist) of sufficient diameter, the dilated vessel wall may collapse immediately after removal of the balloon. This elastic recoil was treated with stent implantation in two cases. Also, the stent was inserted when the balloon dilatation did not expand sufficiently in two cases. A post-procedural fistulography was performed to assess and document the results of the therapy. Following the removal of the vascular sheath, hemostasis was achieved by manual compression.
Primary patency was defined as patency during the interval between primary intervention and repeated radiologic intervention because of dysfunction in permanent hemodialysis vascular access. Secondary patency was defined as patency during the interval between primary intervention and the time when the fistula was surgically declotted, revised, or abandoned; the time when the patient received a renal transplant; death, or the time when the patient was lost to follow-up.
Follow-up of this study ended on September 1, 2009.
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 12.0 statistical software for Windows (SPSS Inc, Chicago, IL, USA). Discrete variables are presented as percentages, and continuous variables, as mean±SD. Standard deviation and frequency analysis was used to evaluate the general characteristics of the patients. Paired t-test and chi-square test was used to determine the significance of the difference between continuous variables. Primary patency rates were calculated according to the Kaplan-Meier life-table analysis. P less than 0.05 was considered statistically significant.
The average number of times the intervention was performed was 1.96 (range, 1-10). The procedure time ranged from 20 minutes to 190 minutes. The mean time from creating the AVF to AVF dysfunction was 13.14±11.58 months.
The general characteristics of the patients are shown in Table 1. The number of patients with diabetes was 71 (60.2%) and without diabetes was 47 (39.8%). Thrombi were found in 57 patients (48.3%). The number of patients with AVF was 65 (55.1%), and with AVG was 53 (44.9%). We performed 122 interventions in 53 patients with AVGs, and 109 interventions in 65 patients with AVFs. In comparison with the AVF group, the AVG group had a higher rate of thrombosis (23.1% vs. 79.2%, p<0.001).
For the 118 patients, the median patency time was 10.45±10.29 months at 92 months of follow-up. The primary patencies for stenotic AVFs at 6, 12, 24, 36, 48, and 60 months were 63.4%, 41.4%, 17.0%, 9.7%, 7.3%, and 2.4%, respectively. The primary patencies for AVGs at 6, 12, 24, and 36 months were 36.9%, 19.5%, 10.8%, 2.1%, respectively, and were obtained by means of the Kaplan-Meier analysis. The results of log-rank test for the Kaplan-Meier analysis of these life-tables revealed significant differences (log-rank=6.42, p<0.05) (Fig. 3). The secondary patency rate was 48.3% at 6 months.
The endovascular techniques used in the interventions and corresponding duration of patency are shown in Table 2. When we compared the duration of patency according to the type of intervention, no statistically significant difference was found (p=0.154).
The median patency time after PTA was 11.0 months and 4.45 months in the non-thrombus and thrombus groups, respectively. Primary patency rates in the non-thrombus group at 6, 12, 24, 36, 48, 60 months were 68.2%, 46.3%, 19.5%, 9.7%, 7.3%, and 2.4%, respectively. Primary patency rates in the thrombus group at 6, 12, 24, 36 months were 32.6%, 15.2%, 8.7%, 2.1% respectively, and were obtained by means of the Kaplan-Meier analysis. Kaplan-Meier analysis of these life-tables revealed significant differences (log-rank=11.95, p<0.001) (Fig. 4).
When we compared the duration of patency between the diabetic and nondiabetic patients, the duration of patency was 9.80 months in the diabetic group and 11.42 months in the nondiabetic group, thereby showing a higher duration of patency in the nondiabetic group (p=0.609). The primary patency rates in the diabetic group at 6, 12, 24, and 36 months were 48%, 32.6%, 11.5%, and 5.7%, respectively, and 51.4%, 25.7%, 17.1%, and 5.7%, respectively, in the nondiabetic group, and were obtained by means of the Kaplan-Meier analysis. There were no statistically significant differences between the groups in the patency rates (log-rank=0.35, p>0.05).
No significant difference was detected in the patency rate between patients with and without urokinase infusion (log-rank=0.00, p>0.05).
The complication rate was 1.73% (4/231; 2 cases of pseudoaneurysms and 2 cases of extravasation). Initial therapy failures were seen in all the AVG thrombus lesions. The failure rate was 2.16% (5/231; >30% residual stenosis after angioplasty). Either new AVF formation or surgical thrombectomy was performed in the cases of failed intervention.
A re-stenosis can be treated radiologically, with or without a stent, or surgically, depending upon the individual situation and the potential invasiveness of the surgical treatment.
The AVF is regarded as the vascular access of choice for hemodialysis because of its superior patency and lower complication rates. Vascular access complications are one of the main causes associated with an increase in morbidity and mortality in stage 5 chronic kidney disease patients.10) Stenosis is considered the major cause of AVF dysfunction. The pathogenesis of venous stenosis is not fully understood. The pathophysiology underlying the occurrence of stenosis is complex. It includes cellular proliferation, microvessel formation, and cytokine expression by smooth muscle cells, endothelial cells, and macrophages.11) The cytokines result in further activation and proliferation of these cell types, ending in venous neointimal hyperplasia.11)
In the past, surgical treatment performing correction of the stenotic lesion using a bypass or a patch was the only option.
Comparison of outcomes of surgical versus percutanuous repair of a dysfunctional fistula is confusing. The cumulative patency rates for AVFs have not been shown to be superior with the use of either method. The success rate of PTA varies in different centres. Surgical repair has been reported to achieve cumulative 1-year primary patency rate of approximately 55%.12)13)
For thrombosed AVFs, a poor patency rate was initially reported; Vorwerk et al.14) reported a primary patency of 50% at 6 months. Overbosch et al.15) reported the median primary patency being 14 weeks. Later, Turmel-Rodrigues et al.16) reported better results, with primary and secondary patency rates (including initial technical failure) at 6 and 12 months of 74% and 81%, and 60% and 81%, respectively. In 2000, Turmel-Rodrigues et al.17) reported primary and secondary 1-year patency rates of 49% and 81%, respectively for failure and thrombosed forearm fistulas. In 2001, Manninen et al.18) reported primary patency rates of 58% at 6 months and 44% at 1 year. In 2003, Kim et al.19) reported primary patency rates of 58.7±5.2% at 6 months, 43.0±6.0% at 1 year and 18.1±6.0% at 2 years. Secondary patency rate of 40.0±8.1% at 2 years was reported. In 2008, in a study by Miquelin et al,7) a total of 22 angioplasties were performed in 20 fistulae of 19 patients. Primary patency was 82.4% over three months; 81.2% over six months; 54.5% over 9 months and 50% over 1 year. In our study, the primary patencies were compared on the basis of thrombosis, diabetic status, endovascular technical methods, AVF and AVG. Lower primary patency rates were seen in AVGs and thrombus groups compared to other studies.
Since 1988, multiple reports have described the use of metallic stents to primarily treat central venous stenoses. Early reports were very optimistic. Landwehr et al.20) treated 7 patients with central vein stenoses with Palmaz stents. They described a 100% technical success rate, but the follow-up period was limited. More recent reports have included longer follow-up and the findings have been more modest. Oderich et al.21) presented 40 central venous obstructions that were treated with 50 stents (mostly self-expanding). Over a mean follow-up of 16 months, primary patency rates of 27% and 9% at 1 and 2 years, and secondary patency rates of 71% and 39%, respectively, were reported. In another recent series by Haage et al.22) 50 patients who underwent stent placement as the primary treatment for central venous obstruction were evaluated. They reported primary patency rates of 56% and 28% at 1 and 2 years, respectively. Disadvantages of stent placement include potential collateral vein obstruction, shortening and migration of the stent, infection, and the loss of outflow in the extremity if the stent should fail.23)24)
In a retrospective comparison of surgical and percutaneous treatment for graft thrombosis, Sands et al.25) performed 75 surgical thrombectomies and 71 percutaneous pharmacomechanical procedures and observed no significant differences in success rates, primary patencies, and rate of complications.
Although PTA and surgery have comparable initial success rates, recurrence is invariably more frequent after the former.26)27) The treating physician must be prepared for repeat interventions, sometimes multiple, over the months and years after the initial declotting procedure. According to Haage,28) 48 episodes of re-stenosis and 27 episodes of re-occlusion were noted in 54 hemodialysis patients, amounting to an average of 1.4 re-interventions per patient in a mean follow-up period of 13 months.
Complications of angioplasty have been reported in about 2-16% of cases, with the most common being immediate venous rupture during the procedure, the formation of pseudoaneurysms, acute thrombosis and periprocedural bacteremia.5)6) Reaction to the contrast has not implicated a higher mortality rate of these patients.5)6)
The current study was conducted in a relatively small number of patients. Therefore, further randomized, large-scale studies are required.
In conclusion, PTA is an effective therapy in restoring the function of failing AVFs.
Figures and Tables
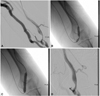 | Fig. 1A severe stenotic lesion. A: stenosis in the anastomotic fistulae between the brachial artery and basilic vein. B: a waist in the 14 mm length balloon is seen. C: percutaneous transluminal angioplasty with 16 mm length balloon. D: post-angioplasty injection shows near resolution of the stenosis. |
 | Fig. 2Thrombosed fistula. A: fistulogram shows a total occlusion involving a thrombus at the arterial anastomosis of a brachial-axillary dialysis graft. B: angiogram shows the balloon inflated at the diseased segment. C: angiogram after ballooning shows insufficient dilatation and residual clots. D: radiograph shows that an Arrow-Trerotola percutaneous thrombolytic device is used at the lesions of clots. E: final angiography shows no residual clots and confirmed an excellent result. |
References
1. Surlan M, Popovic P. The role of interventional radiology in management of patients with end-stage renal disease. Eur J Radiol. 2003. 46:96–114.
2. Haage P, Gunther RW. Radiological intervention to maintain vascular access. Eur J Vasc Endovasc Surg. 2006. 32:84–89.
3. Dougherty MJ, Calligaro KD, Schindler N, Raviola CA, Ntoso A. Endovascular versus surgical treatment for thrombosed hemodialysis grafts: a prospective, randomized study. J Vasc Surg. 1999. 30:1016–1023.
4. Guerra A, Raynaud A, Beyssen B, Pagny JY, Sapoval M, Angel C. Arterial percutaneous angioplasty in upper limbs with vascular access devices for haemodialysis. Nephrol Dial Transplant. 2002. 17:843–851.
5. Tang S, Lo CY, Tso WK, Li FK, Chan TM. Percutaneous transluminal angioplasty for stenosis of arteriovenous fistulae: a review of local experience. Hong Kong Med J. 1998. 4:36–41.
6. Coskun M, Boyvat F, Kurt A, Agildere AM, Niron EA, Bilgin N. Percutaneous balloon angioplasty for permanent hemodialysis with direct arteriovenous fistulae. Transplant Proc. 1998. 30:816–818.
7. Miquelin DG, Reis LF, da Silva AA, de Godoy JM. Percutaneous transluminal angioplasty in the treatment of stenosis of arteriovenous fistulae for hemodialysis. Int Arch Med. 2008. 1:16.
8. Lahoche A, Beregi JP, Kherbek K, Willoteaux S, Desmoucelle F, Foulard M. Percutaneous angioplasty of arteriovenous (Brescia-Cimino) fistulae in children. Pediatr Nephrol. 1997. 11:468–472.
9. Probst P, Mahler F, Krneta A, Descoeudres C. Percutaneous transluminal dilatation for restoration of angioaccess in chronic hemodialysis patients. Cardiovasc Intervent Radiol. 1982. 5:257–259.
10. Campos RP, Do Nascimento MM, Chula DC, Do Nascimento DE, Riella MC. Stenosis in hemodialysis arteriovenous fistula: evaluation and treatment. Hemodial Int. 2006. 10:152–161.
11. Roy-Chaudhury P, Kelly BS, Miller MA, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001. 59:2325–2334.
12. Oakes D, Sherck J, Cobb L. Surgical salvage of failed radiocephalic ateriovenous fistulae: techniques and results in 29 patients. Kidney Int. 1998. 53:480–487.
13. Hodges TC, Fillinger MF, Zwolak RM, Walsh DB, Bech F, Cronenwett JL. Longitudinal comparison of dialysis access methods: risk factors for failure. J Vasc Surg. 1997. 26:1009–1019.
14. Vorwerk D, Schurmann K, Muller-Leisse C, et al. Hydrodynamic thrombectomy of haemodialysis grafts and fistulae: results of 51 procedures. Nephrol Dial Transplant. 1996. 11:1058–1064.
15. Overbosch EH, Pattynama PM, Aarts HJ, Schultze Kook LJ, Hermans J, Reekers JA. Occluded hemodialysis shunts: Dutch multicenter experience with the hydrolyser catheter. Radiology. 1996. 201:485–488.
16. Turmel-Rodrigues L, Sapoval M, Pengloan J, et al. Manual thromboaspiration and dilation of thrombosed dialysis access: mid-term results of a simple concept. J Vasc Interv Radiol. 1997. 8:813–824.
17. Turmel-Rodrigues L, Pengloan J, Rodrigue H, et al. Treatment of failed native arteriovenous fistulae for hemodialysis by interventional radiology. Kidney Int. 2000. 57:1124–1140.
18. Manninen HI, Kaukanen ET, Ikaheimo R, et al. Brachial arterial access: endovascular treatment of failing Brescia-Cimino hemodialysis fistulas: initial success and long-term results. Radiology. 2001. 218:711–718.
19. Kim JH, Do YS, Shin SW, et al. Percutaneous intervention for permanent hemodialysis access. J Korean Radiol Soc. 2003. 48:29–37.
20. Landwehr P, Lackner K, Gotz R. Dilatation and balloon-expandable stents for the treatment of central venous stenosis in dialysis patients. Rofo. 1990. 153:239–245.
21. Oderich GS, Treiman GS, Schneider P, Bhirangi K. Stent placement for treatment of central and peripheral venous obstruction: a long-term multi-institutional experience. J Vasc Surg. 2000. 32:760–769.
22. Haage P, Vorwerk D, Piroth W, Schuermann K, Guenther RW. Treatment of hemodialysis-related central venous stenosis or occlusion: results of primary Wallstent placement and follow-up in 50 patients. Radiology. 1999. 212:175–180.
23. Verstandig AG, Bloom AI, Sasson T, Haviv YS, Rubinger D. Shortening and migration of Wallstents after stenting of central venous stenoses in hemodialysis patients. Cardiovasc Intervent Radiol. 2003. 26:58–64.
24. Pruitt A, Dodson TF, Najibi S, et al. Distal septic emboli and fatal brachiocephalic artery mycotic pseudoaneurysm as a complication of stenting. J Vasc Surg. 2002. 36:625–628.
25. Sands JJ, Patel S, Plaviak DJ, Miranda CL. Pharmacomechanical thrombolysis with urokinase for treatment of thrombosed hemodialysis access grafts. ASAIO J. 1994. 40:M886–M888.
26. Beathard GA. Percutaneous transvenous angioplasty in the treatment of vascular access stenosis. Kidney Int. 1992. 42:1390–1397.
27. Schwab SJ, Raymond JR, Saeed M, Newman GE, Dennis PA, Bollinger RR. Prevention of hemodialysis fistula thrombosis. Early detection of venous stenoses. Kidney Int. 1989. 36:707–711.
28. Haage P, Vorwerk D, Wildberger JE, Piroth W, Schürmann K, Günther RW. Percutaneous treatment of thrombosed primary arteriovenous hemodialysis access fistulae. Kidney Int. 2000. 57:1169–1175.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download