Abstract
Ischemic stroke is a common complication of infective endocarditis (IE), occurring in 20-40% of left side IE cases. In these subsets, anticoagulation therapy may provoke hemorrhagic transformation (HT) of ischemic stroke, and complications of this magnitude deteriorate the clinical course for IE patients. However, in cases of IE complicated with a mechanical prosthetic valve, the physician can be concerned over the maintenance of anticoagulation due to the risk of thrombotic complication. According to our retrospective review, HT of ischemic stroke in prosthetic valve endocarditis occurred 13.8% (4/29) of the time in a variety of situations. Some of these even arose in patients with a subtherapeutic range of prothrombin time.
In patients with native valve infective endocarditis (IE), anticoagulation is not recommended because its benefits have never been convincingly demonstrated.1)2) On the other hand, in prosthetic valve endocarditis (PVE), continuation of anticoagulation to prevent thrombotic complications is sometimes recommended.3)
However, in specific conditions such as PVE caused by Staphylococcus aureus (S. aureus) infections with a recent embolic event in the central nervous system (CNS), the general advice is to discontinue all forms of anticoagulation.4) Since S. aureus induced PVE results in a higher overall mortality rate than PVE caused by other microorganisms, anticoagulation should be discontinued for at least the first two weeks of antibiotic therapy. The causes of death are frequently related to CNS complications and in particular, brain hemorrhage. The two week period is required for thrombus organization, preventing acute hemorrhagic transformation (HT) of embolic lesions. Reintroduction of anticoagulation in these patients is recommended with caution, and prothrombin time (PT) needs to be monitored carefully.1)
HT of acute ischemic stroke is an undesirable complication that occurs in 2.2-44% of clinical cases and in up to 70% of pathologic specimens of stroke.5)6) The location, size, and cause of stroke can influence the development of this complication and the use of all antithrombotics. Anticoagulant and thrombolytic agents especially increase the likelihood of serious HT.6-8) Management of patients with HT depends on the amount of bleeding and symptoms, and may include clot evacuation in deteriorating patients.
Since HT may aggravate the extent of disability and the overall prognosis of stroke patients, clinicians have a tendency to become ambivalent about the maintenance of anticoagulation in the cases of ischemic stroke in PVE.9-12) In spite of the treatment recommendation on PVE caused by S. aureus, there is no general consensus about treatment for different pathogens, or when PVE is complicated with ischemic stroke.13-16)
Therefore, we investigated the incidence and clinical features of HT by reviewing IE cases between 2000 to 2010 and evaluated the significance of anticoagulation therapy in PVE with ischemic stroke.
A 72-year-old woman with a mechanical mitral valve was admitted to our institution with symptoms of ongoing fever and drowsiness. She had undergone mitral valve replacement surgery 16 years ago due to severe rheumatic mitral stenosis. Echocardiography revealed vegetation at the mechanical valve and her blood culture showed coagulase-negative Staphylococcus species. Brain magnetic resonance imaging (MRI) revealed multiple infarct lesions which were suspected of having embolic origin (Fig. 1A and B). Even though the patient had previously experienced an embolic stroke, anticoagulation therapy with heparin and warfarin was started and maintained, since her initial international normalized ratio (INR) was 1.18. On day 9 of hospitalization, when her INR was measured as 2.24, her mental state changed suddenly and non-contrast brain computed tomography (CT) was immediately performed. The CT revealed HT of the previous infarct lesion with a midline shifting effect due to a massive hemorrhage (Fig. 1C and D). Due to persistent vegetation and uncontrolled fever, the attending physician considered surgical intervention to control her PVE. However, her general condition deteriorated rapidly after HT and the risk of surgical mortality was too high to attempt the operation. Therefore, she was unable to undergo surgical intervention. After that, her fever remained uncontrolled, and she did not recover her mental state at all. Eventually, on her day 42 of hospitalization, the physician referred her to the geriatric hospital for the purpose of hopeless discharge.
A 58-year-old woman, who had undergone a dual valve replacement surgery four years previously, visited the emergency room with a persistent fever for three days. Initial transthoracic echocardiography revealed mobile vegetations at both mechanical valves and the blood culture revealed the presence of Enterococcus faecalis (E. faecalis). Since her initial INR was 4.15, higher than the target range, anticoagulation therapy was withheld and the INR levels were measured periodically. Despite discontinuing anticoagulation, her INR remained higher than normal, although lower than the therapeutic range. On day 14 of hospitalization, INR was 1.93 and a brain CT was performed to evaluate the source of a persistent headache which had lasted for a day. The CT revealed acute infarction with hemorrhage in the right temporoparietal lobe, so anticoagulation therapy was witheld. Her blood pressure was stable at 110/70 mmHg before and after her headache symptoms. Despite the use of the appropriate antibiotics, the vegetations persisted after systemic embolization (multiple liver, spleen and kidney infarctions were detected by the abdominal CT) and intractable fever continued. Therefore, on day 35 of hospitalization, the patient underwent redo-dual valve replacement surgery, but on day 3 after the operation, she died due to intractable low blood pressure which did not respond to any inotropic therapies.
A 46-year-old woman who had undergone mitral valve replacement 23 years previously was admitted to hospital due to abdominal pain and drowsiness. Her abdominal CT revealed multiple liver and spleen infarctions and transthoracic echocardiography revealed vegetations of the mechanical mitral valve. Blood culture showed the presence of S. aureus. Her INR at admission was 1.43 (subtherapeutic level) and blood pressure was 108/64 mmHg. Her brain MRI revealed multifocal acute infarctions with HT at the bilateral cerebellum and occipital area. To prevent the aggravation of HT, anticoagulation was withheld for five weeks and restarted with caution. The appropriate antibiotic treatment, considering culture susceptibility, was maintained for six weeks and her fever had subsided two weeks after antibiotics initiation. Six weeks follow-up echocardiography revealed disappearance of the previous vegetation, so antibiotic therapy was accordingly stopped. Although her PVE had been treated, the multifocal stroke with HT resulted in severe functional disability in walking, standing, manual activity and speech. Thus she needed a further hospitalization period of approximately 10 weeks for rehabilitation therapy. Although 8 months have passed since her discharge, she is still currently undergoing rehabilitation therapy in an outpatient clinic.
We retrospectively reviewed the histories of 259 patients who had been admitted to our hospital with IE from January 2000 to April 2010 (Fig. 2). Among them, 29 patients (11.2%) had PVE and 8 (27.6%) of the 29 patients suffered from ischemic stroke related to PVE. HT was observed in 4 of the 8 stroke cases. The presence of ischemic stroke and the development of HT were diagnosed by an imaging study in symptomatic patients. Since most clinicians did not order brain imaging studies at the time when patients showed no symptoms for CNS complication, asymptomatic hidden ischemic stroke or HT may have been overlooked.
Differences in clinical features between ischemic stroke with or without HT are presented in Table 1 and 2. The mean INR was higher in ischemic stroke with HT, but due to the small number of cases, was not statistically significant. In-hospital mortality was quite high, up to 50% in ischemic stroke with HT, and the causative pathogens were diverse both in strokes with or without HT (Table 1).
Two out of eight patients passed away and both had HT. The causes of death were uncontrolled infection and aggravated heart failure. Five out of eight undertook redo-valve replacement surgery due to valve dehiscence, a recurrent embolic event, development of tissue defects via infection progression, paravalvular leakage, and growth of vegetation in spite of optimal antibiotic therapies. Four of these five patients were with ischemic stroke only, and the remaining one patient was complicated with HT. As mentioned earlier in Case 2, this patient passed away three days after the operation due to intractable low blood pressure.
In Case 1, HT aggravated the patient's general condition and made her unable to undergo surgical intervention. Although her initial INR was too low for a mechanical mitral valve, anticoagulation therapy in an embolic stroke may act as an aggravating factor for HT. Since the causative pathogen was not S. aureus, there was no specific guideline or consensus about anticoagulation therapy in PVE complicated with embolic stroke for this patient.
In Case 2, since the initial INR was beyond therapeutic level, anticoagulation therapy was withheld continuously. Although INR was within subtherapeutic range at the time of the embolic stroke, HT occurred concurrently. In this case, E. faecalis caused PVE and no anticoagulant was used. Therefore, the discontinuation of anticoagulation does not seem to be enough to prevent the occurrence of HT.
The last patient's case (Case 3) was already complicated by stroke with HT upon admission, and her initial INR was 1.43 in spite of oral anticoagulation. In this case, because her INR was not high, some factor other than clotting tendency must be attributable for HT. Although the causative pathogen was S. aureus, since the time of HT was assumed to be earlier than admission, it seemed impossible to adjust any risk factors that might influence the occurrence of HT.
There are several reports on the effect of anticoagulation in neurologic complications of PVE. Davenport and Hart9) tried to determine the relationship between anticoagulation therapy and neurologic complication in 61 patients with PVE. In his study, 11 patients (18%) suffered an embolic stroke and 6 patients (8%) suffered brain hemorrhage due to septic arteritis, brain infarction, or undetermined causes. No protective effect of anticoagulation therapy with warfarin was observed and no specific risk of hemorrhagic stroke was evident with anticoagulation therapy. However, bioprosthetic valves (25 patients) were included in the analysis, and specific attention to HT in stroke was not given.
In our series, we have attempted to focus on the development of HT and related factors. In summary, the incidence of HT was quite high (50%, 4 out of 8 cases of PVE complicated by ischemic stroke) and the prognosis was very poor (50% mortality, and functional disabilities in survivors). HT was observed along with diverse pathogens other than S. aureus. Although the number of cases was too small to accurately determine a trend, HT could also occur in patients with subtherapeutic INR and normal blood pressure. Thus, at present, the prediction and prevention of HT is very challenging. For optimal care of PVE patients with ischemic stroke, guidelines for anticoagulation therapy should be implemented by conducting further research.
Figures and Tables
Fig. 1
Brain magnetic resonance imaging on day 3 of hospitalization revealed multiple acute infarcts on diffusion-weighted images, suggesting an embolic origin (A and B). Non-contrast brain computed tomography on day 10 of hospitalization revealed hemorrhagic transformation of the previous embolic infarct at the left temporoparietal area and subfalcial herniation due to the mass effect of the hemorrhage (C and D).
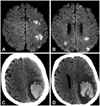
Table 1
Ischemic stroke occurrence in prosthetic valve endocarditis

*Time interval in relation to Day 1, day of hospital admission, †Values which were measured at the day of hemorrhagic transformation detection. PV: prosthetic valve, AV: aortic valve, MV: mitral valve, HT: hemorrhagic transformation, Plt: platelet count, INR: international normalized ratio, BP: blood pressure, CNSA: coagulase-negative Staphylococcus, Lt: left
References
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis diagnosis, antimicrobial therapy, and management of complications. Circulation. 2005. 111:e394–e434.
2. Nishimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis. Circulation. 2008. 118:887–896.
3. Salem DN, Daudelin DH, Levine HJ, Pauker SG, Eckman MH, Riff J. Antithrombotic therapy in valvular heart disease. Chest. 2001. 119:207S–219S.
4. Tornos P, Almirante B, Mirabet S, Permanyer G, Pahissa A, Soler-Soler J. Infective endocarditis due to Staphylococcus aureus: deleterious effect of anticoagulant therapy. Arch Intern Med. 1999. 159:473–475.
5. Okada Y, Yamaguchi T, Minematsu K, et al. Hemorrhagic transformation in cerebral embolism. Stroke. 1989. 20:598–603.
6. Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007. 38:1655–1711.
7. Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke, to bridge or not to bridge? Arch Neurol. 2008. 65:1169–1173.
8. Aviv RI, d'Esterre CD, Murphy BD, et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology. 2009. 250:867–877.
9. Davenport J, Hart RG. Prosthetic valve endocarditis 1976-1987: antibiotics, anticoagulation, and stroke. Stroke. 1990. 21:993–999.
10. Piechowski-Jóźwiak B, Bogousslavsky J. Infectious endocarditis and stroke: any lessons learned since William Osler's Gulstonian lectures? Neurology. 2003. 61:1324–1325.
11. Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) Cohort. Stroke. 1999. 30:2280–2284.
12. Ruttmann E, Willeit J, Ulmer H, et al. Neurological outcome of septic cardioembolic stroke after infective endocarditis. Stroke. 2006. 37:2094–2099.
13. Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: a meta-analysis of randomized controlled trials. Stroke. 2007. 38:423–430.
14. Ryu WS, Kim CH, Kim JH, et al. Prosthetic valve endocarditis. Korean Circ J. 1984. 14:29–36.
15. Kim JS, Kim YJ, Moon KS, et al. Clinical observation of infective endocarditis. Korean Circ J. 2000. 30:166–173.
16. Derex R, Bonnefoy E, Delahaye F. Impact of stroke on therapeutic decision making in infective endocarditis. J Neurol. 2010. 257:315–321.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download